AMAZON multi-meters discounts AMAZON oscilloscope discounts
1. Introduction
The study of electricity, as a human endeavor, encompasses a vast amount of widely diversified material. It reaches into communications, industrial processes of all varieties, transportation and transportation control, scientific research, and military systems of every imaginable type.
Despite its ramifications and complexities, electricity has its beginnings in a few relatively simple concepts. Like every other science, it did not begin to yield its secrets to human research until this research began to take on a semblance of quantitative-ness. For a study to be quantitative all its basic concepts must, first of all, be expressible in numbers which are measured in units having definitions that are rigidly specific. The statement "the trip from city X to city Y is a long one" is completely uninformative because the word "long" is a relative quantity having no intrinsic numerical value.
There is just as little information contained in the statement that the "current flowing from terminal X to terminal Y in an electric circuit is large." To the power engineer a "large" current may be one that can fuse a one-inch-diameter copper bus bar; a radio-tube engineer may consider a current one-millionth this size to be a large one.
The development and comprehension of electrical units of measure properly begin in electrostatics, the study of electric charge at rest. It is here that the forces between electric particles can be ob served experimentally and measured before motion begins, and that the fundamental laws that govern these forces can be derived and stated.
2. Atomic Structure
After the work of Rutherford, Bohr, and their contemporaries, the atom was for many years considered to contain only three particles: electrons, protons, and neutrons. Today the great atom smashers reveal the presence of numerous other subatomic particles, which appear and disappear in atomic interactions. The existence of positrons, pi mesons, tau mesons, kappa mesons, neutrinos, and many others is no longer questionable, and new particles are being brought to light yearly. Apart from knowing that these new particles exist, however, the student of electricity need not concern him self with them. Virtually every phase of this wide subject permits description in terms of electrons and protons only. (Neutrons enter the picture more or less incidentally.) Electrons exert forces on each other. If they are free to move, a group of them will scatter almost instantaneously in a conducting material, so that they become as far apart as possible. This is taken as evidence that a force of repulsion exists between them. Similarly, protons repel each other. Adjacent electrons and protons, however, display a mutual attraction.
The existence of two distinct kinds of forces leads to the conclusion that there are two kinds of electrification or charge. On a purely arbitrary basis, any substance that is repelled by a glass rod that has been rubbed with silk is said to be positively charged. When an object is repelled by a hard-rubber rod that has been rubbed with fur, it is considered negatively charged. These terms have no significance other than that of being opposite; there is no inherent meaning in "negative" or "positive" aside from the fact that bodies bearing these types of electrification behave differently.
A neutral atom contains equal numbers of electrons and pro tons. The condition of neutrality implies a kind of electrical cancellation, in which it is considered that the strength of the proton's charge is exactly equal and opposite to the charge on the electron.
On the basis of the arbitrary choice made above, a proton is designated as a positive charge and an electron as a negative charge.
Although the picture of an atom as a miniature solar system in which the protons and neutrons form the nucleus or "sun," and the electrons are compared to planets orbiting around the nucleus is no longer considered rigorous enough in atomic research, it still suffices for basic electrical study. An atom of argon pictured in this way is given in Fig. 1.
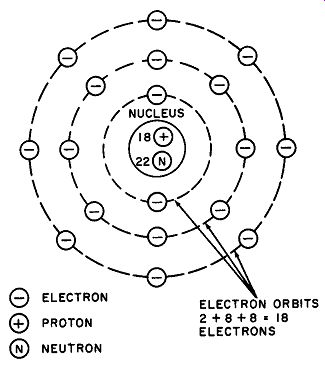
Fig. 1. An atom may be pictured as a nucleus consisting of protons and neutrons,
with electrons revolving about them in fixed orbits. An atom of argon is shown.
There is ample experimental and theoretical evidence now available that proves that the massive particles of the atom are all contained in the nucleus; that protons and neutrons possess approximately the same mass; and that an electron behaves as though its mass were approximately 1/1840 of a proton. Small electrical forces applied directly to the atom are, therefore, much more likely to move electrons rather than the more massive particles. The greater the mass of a particle, the greater is its inertia or tendency to resist a change in motion. Hence electrons are mobile, while protons and neutrons are ordinarily pictured as static under ordinary electro static forces.
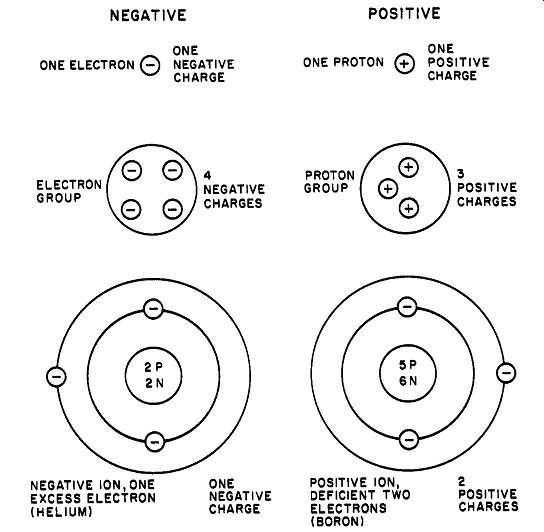
Fig. 2. Some type, of electrically charged particle.
Thus stresses placed on the structure of a given group of atoms are much more likely to displace electrons from their orbits rather than affect the nucleus. This idea is found to be quite valid, because electrons may move from atom to atom freely in certain materials under the influence of electrical forces. If, for example, one electron wanders from its orbit around the nucleus of an argon atom, the condition of neutrality no longer exists and the atom now has a net positive charge. Such a charged atom is called an ion (in this case a positive ion). The roving electron may later attach itself to a neutral atom and establish a net negative charge on it, thus creating a negative ion.
3. Conductors and Insulators
In most substances, particularly non-metals like rubber, plastics, and ceramics, the orbiting electrons are very closely bound to the nucleus, and it is difficult to separate even one electron from the rest of the structure. An electric current is a movement of electrons and, since this movement is impeded in the materials under consideration, such substances are termed nonconductors or insulators. No substance is a perfect insulator, but the current that the usual insulator will conduct with the application of a given electrical force is extremely small compared to that for conductors under the same conditions.
All metals and some non-metals such as carbon and silicon contain many mobile free electrons. These electrons pass from atom to atom under the impetus of even small electrical forces. Because materials of this type permit an easy passage of electrons, they are called conductors. The reader is reminded that both terms-conductor and nonconductor-are relative. Silver is the best known conductor and, compared to it, iron is a very poor conductor indeed. Yet iron con ducts electrons so much better than, say, wood, that it is still referred to as a conductor, whereas wood is called an insulator.
4. The Law of Electric Charges
For introductory study in electrostatics, it is satisfactory to consider an electric charge as a "disembodied" entity that bears either an excess or deficiency of electrons. Because an atom is electrically neutral (contains equal numbers of positive and negative particles), an electric charge may be any one of the following (see Fig. 2): An electron-a single negative charge A proton-a single positive charge A group of electrons-equal in charge to the number of electrons present; negative charge.
A group of protons-equal in charge to the number of protons in the group; positive charge.
A negative ion-an atom that has gained electrons, bearing a negative charge equal to the excess electrons.
A positive ion-an atom that has lost electrons, bearing a positive charge equal to the deficiency of electrons.
Mention has been made before of the effect these charges have upon each other. This may be formally stated as the Law of Electric Charges: Like charges repel each other; unlike charges attract each other.
It is generally conceded that these forces of attraction and repulsion have a greater effect upon electrons than upon positive charges of any type, due to the low mass of the electron and its greater mobility. On the other hand, even positive charges are subject to acceleration under the action of electric forces, particularly when these charges are either static or in motion in free space. (A stream of positive ions may undergo a deflection in path when acted upon by electric forces; the extent of the deflection is less marked than it would be if the same forces were applied to a stream of electrons, but it is measurable.)
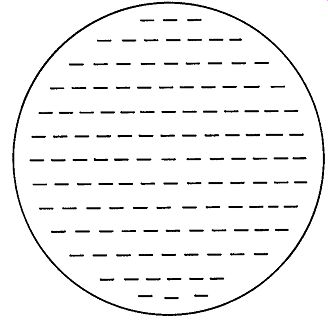
Fig. 3. A charged plate, showing even distribution of the charges on it.
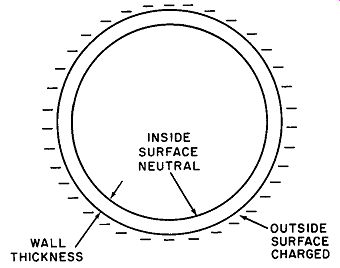
Fig. 4. An electric charge exists only on the outside surface of a charged
sphere.
The Law of Electric Charges may be extended to explain several important phenomena. Assume that a concentrated negative charge has been placed on a small metal disc with an insulated handle (a so-called "proof plane") and that the disc is touched to a large aluminum plate mounted on four insulated legs. When the plate is tested with an electrostatic detector, it is found that the original charge on the proof plane has in large part been transferred to the aluminum plate, and that the charge has spread out evenly over the larger surface, as shown in Fig. 3.
This distribution is explained by the conductivity of the aluminum and the mutual repulsion of the negative charges.
Another example deserving note is the condition of charge of a hollow sphere having a finite and measurable wall thickness. Al though the sphere is made of conducting material, the entire charge is found to be present on the outside surface. The inner surface is completely neutral.
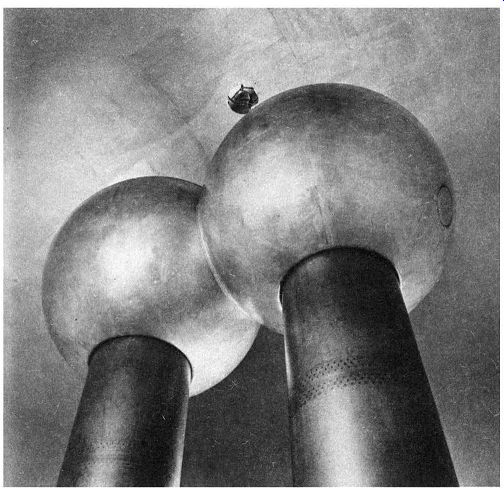
Fig. 5, Van de Graaff 2,000,000-volt generator. MIT, Fig. 6. Gold-leaf electroscope.
A sphere in cross section may be pictured as two concentric circles, as shown in Fig. 4. The mutual repulsion of like charges compels the electrons or ions to move as far apart as possible. In the case of the sphere, the outer surface represents the greatest physical separation. This phenomenon is utilized in safeguarding the operating personnel of large Van de Graaff static generators such as those used for atom-smashing; it is a startling fact that the safest area for the operators is right inside the huge sphere where millions of volts ate being generated. (See Fig. 5.)
5. Electrostatic Detection Equipment
The equipment with which electrical phenomena are detected and measured rarely gives any indication of the processes that occur. Light passing through a lens is easily visible; the size of a basketball bladder changes as compressed air is pumped into it, but electricity is invisible and has no measurable weight. The terminals of a large capacitor appear the same whether it is charged to a lethal potential or not. The task set for electrostatic instruments is that of making electricity evident to our senses.
The gold-leaf electroscope (Fig. 6) is such an instrument. Essentially, the electroscope consists of a glass flask in which a metal rod is supported by passing it through the center of an insulating stopper. The rod is topped by a spherical conductor; suspended from the bottom are two pieces of thin gold foil, extremely small in mass and measuring about ¼ inch by 1 inch. Often, the lower section of the flask is lined inside with tin or aluminum foil.
If a proof plane bearing a positive charge (i.e., a deficiency of electrons) is touched to the top ball, the gold leaves immediately diverge. The extent of the divergence in a given instrument is a function of the magnitude of the original charge on the proof plane.
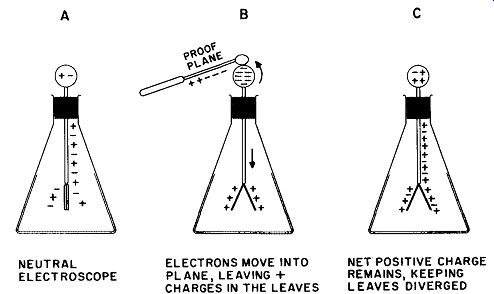
Fig. 7. Charging an electroscope by contact. The charge on the leaves is the
same as that of the charge applied.
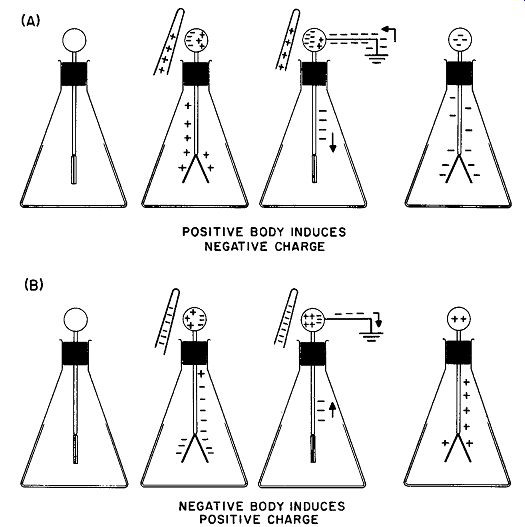
Fig. 8. Charging an electroscope by induction. The charge on the leaves is
opposite to that of the charge applied.
In a neutral electroscope, the atoms, positive ions, and "free" electrons have distributed uniformly over all conducting surfaces and, hence, leave no "net" charge in the absence of an outside charge.
Upon the approach and contact of the proof plane, however, the negative particles (electrons) are drawn up into the sphere. Because the plane and the sphere make contact, the electrons are further drawn into the plane and neutralize (to some degree) the positive charge on it. When the proof plane is removed, the system is left with a deficiency of electrons, and thus has a net positive charge. This sequence is shown in Fig. 7.
In its charged state, the electroscope may be used to detect the presence of charges of either sign. Should a negative body approach a positively charged electroscope, such as that of Figure 7C, residual electrons in the system will be driven down into the gold leaves, partially neutralizing the + charge on them. The divergence of the leaves will diminish, signifying that the charge on the approaching body is negative. Upon the approach of a positively charged body, however, the leaves will diverge still further, because additional electrons will be drawn upward away from the leaves, making the net positive charge greater than it was before.
The process shown in Fig. 7 is called charging by contact. It always results in the appearance of a charge on the electroscope leaves having the same sign as the charging body. It is also possible to place a charge of opposite sign on any body by using the method of induction. This process is shown in Fig. 8. Assume that a positively charged body, such as a rod of glass rubbed with silk, is brought near the sphere of a neutral electroscope without touching it. Electrons move into the top sphere by electrostatic attraction of opposite charges and the leaves diverge as a result of the net positive charge left on them by the withdrawal of electrons. The sphere is now touched with the finger; as this is done, the leaves will be seen to collapse. This is caused by electrons entering the system from the earth through the body and the finger. These "free" electrons neutralize the positive charge on the side of the sphere away from the rod and on the leaves, whereas the electrons on the other side of the rod are tightly "bound" by the attraction of the positively charged glass rod.
The finger is then withdrawn. This has no effect on the leaves, because the excess negative charges present in the system are still tightly bound in the sphere by the attraction of the glass rod. When the rod is removed, however, the leaves instantly diverge. The removal of the rod permits the negative charges to distribute them selves throughout the metallic portions of the electroscope. Because negative charges entered the system via the finger-to-earth connection, the electroscope is left with a net negative charge. Thus, when charged by induction, the electroscope receives electricity of the opposite sign from that of the inducing charge.
A similar sequence takes place when a negatively charged rubber rod is brought near the ball of the electroscope. The steps in this process are shown in Fig. 8B.
6. The Tribo-electric Series
As early as 600 B.C., the Greeks observed that a piece of amber when rubbed against their clothes acquired the property of attracting small pieces of parchment and lint. Although this effect was associated only with amber for a long period of time, it was ultimately found that other substances also possessed this property.
A glass rod and a stick of sealing wax after being rubbed with the same piece of silk attract each other. On the other hand, the rod of sealing wax repels a block of sulfur that has also been rubbed with silk. It appears that the glass gives off electrons to the silk, and the silk to the sealing wax. The glass is positively charged, the sealing wax negatively charged, and they attract each other. The silk, however, gives off electrons to the sulfur as well as to the wax; therefore, the sulfur and wax repel each other. Which of two bodies will become positively electrified by friction depends upon the particular condition of its surface, and depends upon its molecular nature. These ideas have led to the development of a table based on experiment called the triboelectric series. In general, if any two bodies in the table are brought into intimate contact, the one that appears first in the table takes on the positive charge.
THE TRIBOELECTRIC SERIES
1. asbestos
2. glass
3. mica
4. wool
5. cat's fur
6. silk
7. cotton
8. resin, sealing wax
9. hard rubber
10. sulfur
This list, which originated with Michael Faraday, was based upon rubbing contact between substances. There is some indication in the results of current experiments that electrification comes not from friction but simply from intimate contact between materials.
7. Coulomb's Law
Newton's Law of Universal Gravitation first revealed the in verse square characteristics of gravitational force fields. The statement of the law includes the measurable fact that the force between two particles due to a field of force between them always varies inversely as the square of the distance between them. Although Newton did not realize the universality of this concept, a better appreciation of it was brought about when Coulomb, almost 100 years later, discovered that the same rule applied to static charges of electricity and magnetic poles as to masses in gravitational fields.
Using an extremely delicate and precise instrument called a torsion balance, Coulomb determined that:
The force between two charged bodies that are small compared to the distance between them is directly proportional to the product of their charge magnitudes and inversely proportional to the square of the distance between them.
Expressed algebraically, Coulomb's Law reads:
qq' F= Kr2 (1)
…where F is the force of attraction or repulsion between the charged bodies, K is a constant to be discussed shortly, and r is the distance between the centers of the two bodies whose charge magnitudes are represented by q and q'. The constant K depends upon two factors: first, upon the units used to express the other quantities and second, upon the nature of the medium between the charged bodies.
A satisfactory comprehension of electrostatic phenomena can be realized only by achieving a thorough understanding of the meanings and relationships of units of measure in this field. The confusion experienced by students of the subject is understandable, since many authors treat units too lightly. For this reason, the under lying standards of accepted unit systems will be studied in the next section.
8. QUIZ
1. Why do electrons rather than protons move when acted upon by electric forces?
2. Discuss an ion in terms of atomic theory; distinguish between negative and positive ions.
ll. According to current atomic theory, what is the real difference between an electrical conductor and an insulator?
4. State the Law of Electric Charges.
5. Explain qualitatively why a concentrated negative charge placed on a conducting surface spreads out so that it is uniformly distributed on the surface,
6. Explain qualitatively why there appears to be zero charge on the inside of a charged conducting sphere.
7. A neutral electroscope is charged by bringing it into contact with a negative rod. What charge will appear on the leaves of the electroscope? Explain.
8. Describe the process in which a neutral electroscope may be charged negatively by means of a positively charged proof plane.
9. What is the triboelectric series? If each of the following pairs of substances are brought into intimate contact, which one of the pair will take on a positive charge? (a) mica and sulfur, (b) cat's fur and glass, (c) cotton and asbestos, (d) hard rubber and wool.
10. State Coulomb's Law. Why is the constant K required in the equation that expresses Coulomb's Law?