AMAZON multi-meters discounts AMAZON oscilloscope discounts

THE FRONT COVER
To disprove the old saying, "You can't judge a book by its cover", much time and thought have been spent to make these lesson covers attractive, informative, and useful. The rapidly expanding fields of electronics are symbolized at the upper left by the popular diagram of an atom. Here the central nucleus is surrounded by rapidly rotating electrons, the energy of which makes possible all modern applications of electricity and electronics.
The training program includes a number of sections or series of lessons each with a distinctive background color. The title, section code letters, and series number of each lesson are shown in the center, while the code letters and series number appear also as a bar across the fold at the left. The position of this bar is lower on each succeeding lesson so that, when the series is filed with only the folded backs visible, the bars form a diagonal line to indicate proper numerical sequence. Thus, the distinctive color of each series and the bar position provide a convenient index for future reference.
ELECTRONS
Contents:
The Electronic Technician
The Technician's Duties
What an Electronic Technician Must Know
Your Technical Training
Electrons
Atoms
Charged Particles
Some people fail because they never begin. More people fail because they never finish. Stick-to-it-ivness wins oftener than genius or luck. You may make mistakes, others may misjudge you. You may be tired and discouraged. But, if you stick, by a by everything and everybody will give way to you.
If you have principles they will do you no go unless you stick to them. If you do not stick to your friends, you don't deserve friends. If you do not stick to your job, you cannot make a success of it and fi a better one. In a troubled and anxious time of the Civil War, General Grant said, "I will fight it out this line if it takes all summer." Let that be your motto.
-Dr. Frank Crane
Electronics is the magic of our century.
At the beginning of the 20th century, electric lights and tele phones were curious toys. Very few had vision enough to use them. During those years, only brave people ventured with their automobiles onto the city streets, and most of these cars were steam driven or powered by storage batteries. Airplanes meant absolutely nothing to the public.
Home entertainment was provided at that time by player pianos and mechanical type phonographs. There were no radio or television broadcasts. Without electricity in the home, there were no refrigerators, deep freeze units, air-conditioners, vacuum cleaners, automatic toasters, and the many other appliances which we take for granted today. Al though Thomas Edison had patented a motion picture camera and projector, there were no movie theatres as we know them now.
Electronics is growing so fast and so big, large numbers of our population are being directly employed by it. The number of radios, over 100,000,000, indicates just how big electronics is today.
In fact, from laboratory models, television has grown rapidly to more than 30,000,000 sets in seven years, and now color television is ready to occupy the American home.
Electronic products and equipment are found nearly every where. Two-way radiotelephone in trucks, taxis, trains, ships and aircraft are in operation not only in the United States but in many nations throughout the world.
Every day, industry is demanding more electronic instruments.
Whether it is a steel mill, an automobile manufacturer, a textile mill, a bakery, a printing plant, or an oil refinery-industry needs electronic equipment to more precisely control quality and speed production.
In fact, practically every type of occupation uses electronic equipment. The doctor, dentist, lawyer, chemist, mechanical engineer, banker, aviator, musician, and dietician all find electronic devices useful in their work.
THE ELECTRONIC TECHNICIAN
The key men in all of this vast growing field are the individuals who understand how the electronic devices work and how to best apply such devices to these in creasing needs.
It takes the help of television, radio, and other electronic technicians to produce the equipment in the laboratory. Other equipment is constructed by the technician for a particular need. Once constructed, a large number of technicians are required to properly install it. Finally, no equipment runs indefinitely without maintenance or repair. This need alone employs many thousands of electronic technicians.
THE TECHNICIAN'S DUTIES
Regardless of what type of equipment you will work on, television, radio receivers, transmitters, industrial control devices, or electronic instruments, and no matter which division of the field you may choose to work in, as an electronic technician you must be able to:
1. Understand how the equipment operates.
2. Recognize when it is operating improperly.
3. Determine the portion of the equipment causing the improper operation.
4. Locate and correct the defect within that portion of the equipment.

--- A modern large screen television receiver controls electrons in a variety
of ways. Courtesy Stewart Warner Corp.
Although a laborer can follow the shop foreman's assembly line instructions, where a faulty operation appears it takes a technician to determine what construction procedure is at fault, for only a trained technician knows what the good procedures are. Many people can read unpacking instructions, but usually it takes a skilled technician to adjust the electronic equipment for best operation.
WHAT AN ELECTRONIC TECHNICIAN MUST KNOW
Electronics is the control of electrons. Therefore, to be a good electronic technician, you must first know something about electrons, where they exist, and the nature of the electron that makes control possible. Then you can learn about various parts and how you use them to control the electron. Next you will learn combinations of the parts, which the technician calls "circuits", that are used to get certain effects.
Finally you see how these circuits are used in the field of your interest to accomplish the desired results in many different types of equipment.
All of this information is the "basic theory", but your know how as a technician is more than just basic theory. You must be able to understand the language and read graphs, charts, and symbols. Also, you should acquire a "finger skill" so that you can easily and neatly wire up complete circuits or replace a faulty part.
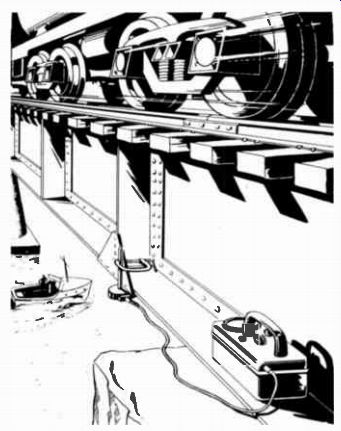
-------- An electronic instrument designed to measure vibration tells whether
or not the bridge may be considered safe. Courtesy The Victoreen Instrument
Co.
Every technical field has its own language. Either the every day words do not have an exact enough meaning, or their meaning is too specific to be used in a technical sense. Therefore, to avoid confusion, words are created and adopted by each field so that exact ideas are expressed.
It is a mighty poor carpenter who does not understand the meanings of terms like "saw horse", "riser", "off plumb", and "countersink". Just as handicapped is the electronic technician who doesn't know the meaning of "ohm", "transformer", "oscillator", or "rectifier". Each needs his technical language--language which you will learn as you advance in your training program.
Again referring to a good carpenter, he needs to understand drawings and architectural blue prints. Not to be able to under stand these would be a serious handicap. Although it might be possible to replace them with a long, wordy description, it is much easier for him when he understands the purpose of each symbol used on the blue print.
The same holds true for the electronic technician. The diagrams are a very valuable aid when learning how the various circuits are connected, but far more important, once you are active in the electronics field you will refer to them daily for easy to locate and accurate information regarding the particular model of equipment with which you are working.

------------ A variety of electric and electron equipment found at a broadcast
station. Courtesy WBAP-Fort Worth, Texas.
Just as the proper manner to hold a chisel or use a saw are little finger skills expected of a carpenter, the correct use of a soldering iron or a meter or the skilled use of long nose pliers and diagonal cutters are needed by electronic technicians. Aside from the basic theory, these little skills do distinguish the successful technician from the sloppy workman.
Finally, there are tricks to every trade. Electronics is no exception. Once the basic theory is understood and finger skills are acquired along with the language and diagram reading abilities, the technician develops procedures which enable him to construct, maintain and locate defects with a minimum of time and effort. In fact, once he has carefully developed this technique it seems to the onlooker that the technician uses magic. It looks so easy.
YOUR TECHNICAL TRAINING
In fact it is easy when learned one step at a time. That is just how this training program works.
In each successive lesson, we describe the electrons, their nature, various parts and how these control electrons, useful combinations

---- Life tests are being run on these television picture tubes. They are
periodically checked by a technician. Courtesy General Electric Company
of these parts, and finally how these combinations are used in a specific piece of equipment.
At the same time you will find out what each symbol is for, learn what each term means, and gain experience in reading dia grams and graphs. Also you will acquire good experience in your laboratory projects. Not only are you becoming skillful in handling your tools, but you will work with circuits and discover what factors determines their proper or improper operation.
Finally, these lessons describe just how to use this knowledge most effectively in your work and with a minimum number of steps.
With these techniques you will be an electronic technician capable of taking decisive steps rather than be a tinkerer stumbling along.
ELECTRONS
But first things must come first. Since all electronic equipment controls electrons, just what is an electron?
Before we tell you what these electrons are and do, you ought to take a look about you. The walls, floor, tables, chairs, etc. all about you are all material objects.
You can see and feel them. Yet, someone might ask you, "What are these materials made of?"
All materials are made of tiny particles called molecules, which are invisible to the naked eye. It has been proven experimentally by a number of people and accepted by the rest of the world.
Millions of these particles go to make up a straight pin.
Electronics is the control of electrons.
Furthermore, these tiny invisible particles are composed of atoms-all material; are composed of atoms. Of what are atoms made? Well, atoms are made of two things: a nucleus and electrons about the nucleus.
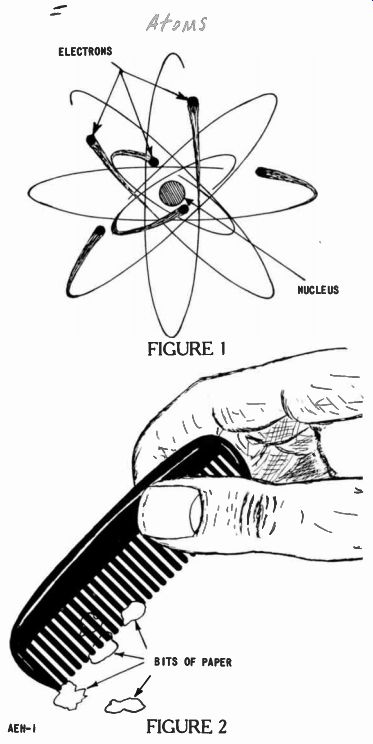
Figure 1 at the back of this book illustrates what the atom may look like.
In the atom, most of the space is empty. As shown, the nucleus is at the center, and electrons are whirling about it. This arrangement may be likened to our solar system. Like the electrons, the earth and other planets revolve about the sun which forms the nucleus of the system. .However, the electrons move much faster than the earth. Their speeds range up to 186,000 miles a second--the speed of light.
When comparing the nucleus and electrons in the atom, although the nucleus is very much smaller than an electron, the electron contributes about as much weight to the atom as does a marble compared to a bowling ball. That is, the electron is ever so much lighter than the very dense nucleus.

--------------- For too small to be seen by the most powerful microscope.
When compared to the size of an electron, the distance between the nucleus and the high speed revolving electrons is tremendous. Use the distance between the earth and the sun, some 93,000,000 miles, as the yardstick.
The relative distance between the nucleus and the revolving electrons is about the same. This is why the atom is mostly empty space as mentioned.
Yet, through this empty space something normally keeps the electrons from leaving their circular path. Some power or "invisible glue" holds them swinging about the nucleus. This "glue" is somewhat like the force that keeps the water in a bucket as it is whirled in a circle.
-------------- Like charges repel.
You'll note six electrons shown in Figure 1. The electrons shaped like a ball are all moving very rapidly about the nucleus. None of the electrons shown bump into each other within an individual atom.
ATOMS
Insofar as anyone knows, all electrons are identical no matter in which atom they appear. That is, electrons in iron atoms are the same as those electrons in gold atoms.

------------- Unlike charges
A question which you may ask now is: why are the various materials so different from each other? To answer this question, we must mention one more thing about atoms. It has been found that the atoms of different materials or substances contain different numbers of electrons. For ex ample, an atom of iron contains 26 electrons, while a gold atom has 79 electrons. An atom of car bon has only 6 electrons, like Fig. 1, and the hydrogen atom is the poorest of all-1 electron. These differences are found in all materials or substances. That is, al though all electrons are alike, they can be grouped together in different numbers to form various atoms.
In addition to having its own particular quantity of electrons, each type of atom has a nucleus which is different from that of all other types of atoms. Thus, each material or substance is different because no two kinds of atoms contain the same number of electrons or the same nucleus.
An atom is the smallest unit in to which substance or any material can be divided without losing the qualities by which we identify it. If we divide an atom by re moving some electrons and part of the nucleus, we have a different material or substance. If we do this to a gold atom, we could get an iron atom. Reverse the process by starting with an iron atom, and adding parts to the nucleus and the proper number of electrons, gold results. However, either process costs a fortune-it requires a tremendous quantity of energy and equipment.
It so happens that there are 100 types of material which can be divided down to atoms. These types of material are called elementary substances, or simply, elements. All of the thousands of other kinds of materials are composed of small particles known as molecules. In turn, these molecules are made up of atoms of two or more of the various elementary substances or elements.
CHARGED PARTICLES
Electrons are small particles of electricity. These particles have a peculiar way of acting toward one another which makes it possible to do the many things that we do with them. If you were to try to bring two electrons close together, you would find that they would resist your action. As soon as you let go of them, they fly away from each other. They resist remaining close together! You would obtain the same results if you tried the experiment with two nuclei from a pair of atoms.
----------------- Shown is a table model AM-FM receiver, one of 100,000,000
radios in the United States. Courtesy Motorola, Inc.
However, if you brought an electron just near enough to a nucleus, you might notice them pulling toward one another. When you let them go, they would fly together. From these actions, you could decide that LIKE PARTICLES OF ELECTRICITY REPEL each other, whereas UNLIKE PARTICLES AT TRACT.
Because of the way they act, electrons and nuclei are considered to be different kinds of electricity. To distinguish between them, we say that a nucleus is POSITIVE electricity, and an electron is NEGATIVE electricity.
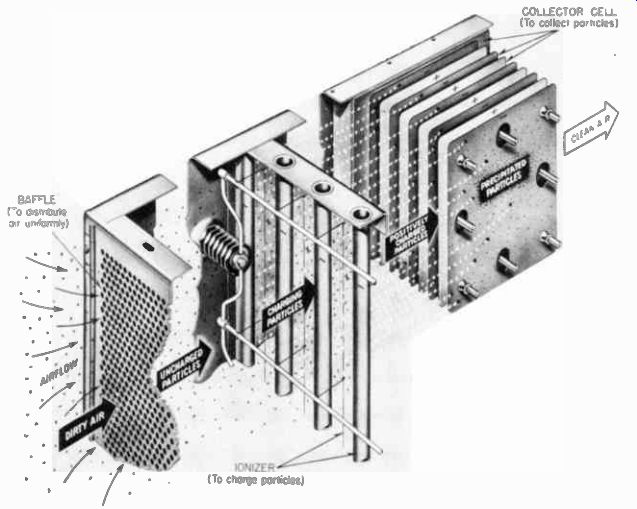
-------- This air cleaner makes use of the fact that when electrons are
removed from atoms on dirt particles, the positive dirt atoms are attracted
by the negative plates in the collector cell. Courtesy Westinghouse Electric
Corp.
In any normal atom, the positive nucleus exactly balances the negative electrons which the atom contains, and its attraction holds them in the atom. Therefore, a normal atom is neither positive nor negative-it is said to be NEUTRAL. However, when the negative electricity in a normal atom is decreased by removing one or more electrons without disturbing the nucleus, then the atom is no longer neutral. The atom contains more positive than negative electricity. On the other hand, when electrons are added to a normal atom by some means, again a condition of unbalance is produced and the atom has an excess of negative electricity.
POSITIVE ATOMS are deficient in electrons; NEGATIVE ATOMS contain an excess of electrons. These charged atoms also play an important part in the operation of electronic equipment.
When electrons are removed from the atoms of an object, the object then has more positive than negative electricity. The entire object is said to be positive. If electrons are added to give an object an oversupply of negative electricity, then the object is said to be negative.
Just like the electrons and nuclei, negative and positive objects attract each other. This attraction can be observed by means of an ordinary pocket comb and some tiny scraps of paper. Run a comb through your hair a few times, or rub it on woolen clothing, then touch it to small scraps of paper. Usually, the bits of pa per will cling to the comb and may be lifted as shown in Figure 2.
The friction caused by rubbing two objects together rubs more electrons off of one material onto the other. Hence, one is charged positive and the other negative.
This little demonstration has an interesting feature. When the comb is charged negative, as it is brought close to the bits of paper it repels a few electrons out of the paper into the surface below.
This leaves the paper positive which is then attracted to the negative comb.
On the other hand, if the comb is positive, it attracts electrons out of the surface into the paper.
Since these excess electrons charge the paper negative, it is again attracted to the positive comb.
The fact that neutral atoms can be converted into positive and negative charged atoms is very important. The comb and the bits of paper illustrate the generation of both kinds of charges. This is evidence that there are such charges. By no means is this the only evidence, but it is something anyone can try.
Now we know that electrons are found in the small particles of all matter called atoms. More over these electrons have very important characteristics: they al ways have the same negative charge and they are always attracted by positive and repelled by negative charges.
It is this important action that is used in every piece of electronic equipment, whether it is your car radio or television receiver or a huge electronic "brain" mentioned so often in the newspapers.
In the next lesson, we use these facts to describe our first method of controlling electrons.
IMPORTANT DEFINITIONS
In this and the following lessons, the important new words are introduced with boldface type in the text and are listed in the back of the lesson for handy reference. As an aid in pronouncing the words, a system of re-spelling is used. In this system, a particular sound is represented by a group of letters, the pronunciation of which closely resembles that of the required sound.
For example, "radio" may be re-spelled as (RAY di oh), "pilot" as (PIGH lught) and DeVry as (Dee VRIGH). To show the accented parts of the words, two methods are employed. The main accent is shown by CAPITALIZING the part to be stressed, as in (RAY di oh). When two parts of the word are to be accented, the capitalized part shows where the main stress should be placed and the italics show where the secondary stress should be placed. An example of a double accented word is "anybody", which is re-spelled as (EN i bah di). In addition to the letters of the alphabet, two additional marks often are employed: the colon ( :) and the apostrophe ('). The colon is used to change the sound of double-o from that of the word foot (foot) to that of the word food (foo :d). In many words, such as lemon, about, and circuit, the unaccented vowels (o, a, and u respectively) often are pronounced as though they were spelled uh, and therefore, this term is used to represent those vowels in the respelling system. Frequently, an apostrophe (') is used to represent the "uh" sound as in (LEM 'n) or (TEL i vizh 'n).
ATOM--A small particle which contains electrons and a nucleus, which is a positively charged center.
ELECTRON--(i LEK trahn)-an extremely small particle which is contained in the atoms of all materials and is negatively charged.
MOLECULE--A particle which contains one or more atoms. Various combinations of atoms in the molecule go to make up all materials in the world as we know them.
STUDENT NOTES
-------
FIGURE 1
FIGURE 2
FROM OUR Director’s NOTEBOOK
LET'S GO
You have now embarked on o new journey that will take you well into the inner realms of Radio and Electronics. The ease and speed with which you reach your goal, will depend upon how you pull on the oars. A steady and constant pull will get you there more quickly and more smoothly, than a few sudden jerks now and then with free drifting in between.
And just as if takes so many strokes of the oars to reach the end, just so a prescribed number of lessons must be completed. Most rapid progress can be made not by trying to do too much of one time and getting tuckered out, but by following o definite schedule and doing a certain number of pages every day--it's that steady pull on the oars that counts.
By knowing more and being able to do your work better, you will quickly make yourself worth more to the world and to your community, and it won't take the world long to find that out. Merit is the one straight road to success. The man who is successful is the man who is useful. Ability and capacity always find opportunity. They never remain undiscovered, because they are sought by too many who ore anxious to We are sure you'll enjoy the trip, and here's wishing you a happy arrival at the port of opportunities lo use them in Electronics.
Yours for success,
- moi.