AMAZON multi-meters discounts AMAZON oscilloscope discounts
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form via nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation. Nucleation of crystalline, amorphous and even vacancy clusters in solid materials is also important, for example to the semiconductor industry. Most nucleation processes are physical, rather than chemical, but a few exceptions do exist (e.g. electrochemical nucleation). A good example would be the famous Diet Coke and Mentos eruption. Nucleation normally occurs at nucleation sites on surfaces contacting the liquid or vapor. Suspended particles or minute bubbles also provide nucleation sites.
This is called heterogeneous nucleation. Nucleation without preferential nucleation sites is homogeneous nucleation. Homogeneous nucleation occurs spontaneously and randomly, but it requires superheating or supercooling of the medium. Nucleation is involved in such processes as cloud seeding and in instruments such as the bubble chamber and the cloud chamber.
Examples of nucleation
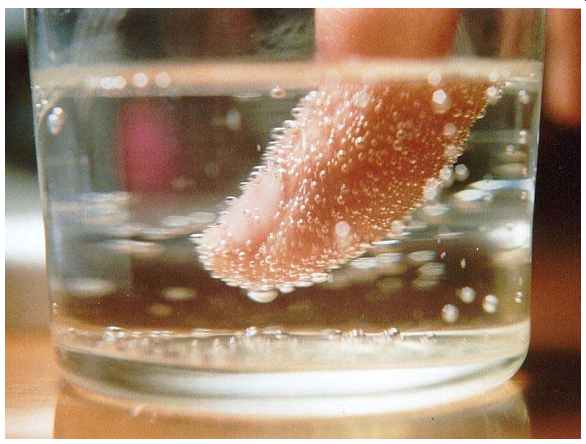
-- Nucleation of carbon dioxide bubbles around a finger
• Pure water freezes at -42°C rather than at its freezing temperature of 0°C if no crystal nuclei, such as dust particles, are present to form an ice nucleus.
• Presence of cloud condensation nuclei is important in meteorology because they are often in short supply in the upper atmosphere.
• All natural and artificial crystallization process (of formation of solid crystals from a homogeneous solution) starts with a nucleation event.
• Bubbles of carbon dioxide nucleate shortly after the pressure is released from a container of carbonated liquid. Nucleation often occurs more easily at a pre existing interface (heterogeneous nucleation), as happens on boiling chips and string used to make rock candy. So-called Diet Coke and Mentos eruptions are a dramatic example.
• Nucleation in boiling can occur in the bulk liquid if the pressure is reduced so that the liquid becomes superheated with respect to the pressure-dependent boiling point. More often nucleation occurs on the heating surface, at nucleation sites.
Typically, nucleation sites are tiny crevices where free gas-liquid surface is maintained or spots on the heating surface with lower wetting properties.
Substantial superheating of a liquid can be achieved after the liquid is de-gassed and if the heating surfaces are clean, smooth and made of materials well wetted by the liquid.
• Nucleation is relevant in the process of crystallization of nanometer sized materials, and plays an important role in atmospheric processes.
• Nucleation is a key concept in polymer, alloy and ceramic systems.
• In chemistry and biophysics, nucleation can also refer to the phaseless formation of multimers which are intermediates in polymerization processes. This sort of process is believed to be the best model for processes such as crystallization and amyloidogenesis.
• In molecular biology, nucleation is used to term the critical stage in the assembly of a polymeric structure, such as a microfilament, at which a small cluster of monomers aggregates in the correct arrangement to initiate rapid polymerization.
For instance, two actin molecules bind weakly, but addition of a third stabilizes the complex. This trimer then adds additional molecules and forms a nucleation site. The nucleation site serves the slow, or lag phase of the polymerization process.
• Some champagne stirrers operate by providing many nucleation sites via high surface area and sharp corners, speeding the release of bubbles and removing carbonation from the wine.
• Sodium acetate heating pads use a metal disk as a nucleation centre for exothermic crystallization Ionizing radiation

-- Radiation hazard symbol.
--- Ionizing radiation hazard symbol (recently introduced).
Ionizing radiation consists of particles or electromagnetic waves energetic enough to detach electrons from atoms or molecules, thus ionizing them. The degree and nature of such ionization depends on the energy of the individual particles or waves, and not on their number. An intense flood of particles or waves will not cause ionization if these particles or waves do not carry enough energy to be ionizing. Roughly speaking, particles or photons with energies above a few electron volts (eV) are ionizing. Ionization produces free radicals, which are atoms or molecules containing unpaired electrons, that tend to be especially chemically reactive due to their electronic structure.
Examples of ionizing particles are alpha particles, beta particles, neutrons, and cosmic rays. The ability of an electromagnetic wave (photons) to ionize an atom or molecule depends on its frequency. Radiation on the short-wavelength end of the electromagnetic spectrum-high frequency ultraviolet, x-rays, and gamma rays-is ionizing. Lower energy radiation, such as visible light, infrared, microwaves, and radio waves, are not ionizing.
The latter types of lower energy electromagnetic radiation may damage molecules, but the effect is generally indistinguishable from the effects of simple heating. Such heating does not produce free radicals until higher temperatures (for example, flame temperatures or "browning" temperatures, and above) are attained. In contrast, damage done by ionizing radiation produces free radicals, even at room temperatures and below, and production of such free radicals is the reason these and other ionizing radiations produce quite different types of chemical effects from (low-temperature) heating. Free radical production is also a primary basis for the particular danger to biological systems of relatively small amounts of ionizing radiation that are far smaller than needed to produce significant heating. Free radicals easily damage DNA, and ionizing radiation may also directly damage DNA by ionizing or breaking DNA molecules.
Ionizing radiation is ubiquitous in the environment, and also comes from radioactive materials, x-ray tubes, and particle accelerators. It is invisible and not directly detectable by human senses, so instruments such as Geiger counters are usually required to detect its presence. In some cases it may lead to secondary emission of visible light upon interaction with matter, as in Cherenkov radiation and radio-luminescence. It has many practical uses in medicine, research, construction, and other areas, but presents a health hazard if used improperly. Exposure to radiation causes damage to living tissue, and high doses can result in mutation, radiation sickness, cancer, and death.
Types
--- Photons interact electromagnetically with charged particles, so photons
of sufficiently high energy also are ionizing. The energy at which this begins
to happen with photons (light) is in the high frequency end of the ultraviolet
region of the electromagnetic spectrum.
Charged particles such as electrons, positrons, muons, protons, alpha particles, and heavy atomic nuclei from accelerators or cosmic rays also interact electromagnetically with electrons of an atom or molecule. Muons contribute to background radiation due to cosmic rays, but by themselves are thought to be of little hazard importance due to their relatively low dose. Pions (another very short-lived sometimes-charged particle) may be produced in large amounts in the largest particle accelerators, but are not a theoretical biological hazard except near such machines, which are in practice are subject to heavy security while in use.
Neutrons, on the other hand, having zero electrical charge, do not interact electromagnetically with electrons, and so they cannot directly cause ionization by this mechanism. However, fast neutrons will interact with the protons in hydrogen (in the manner of a billiard ball hitting another, head on, sending it away with all of the first ball's energy of motion), and this mechanism produces proton radiation (fast protons). These protons are ionizing because they are charged, and interact with the electrons in matter.
A neutron can also interact with other atomic nuclei, depending on the nucleus and the neutron's velocity; these reactions happen with fast neutrons and slow neutrons, depending on the situation. Neutron interactions in this manner often produce radioactive nuclei, which produce ionizing radiation when they decay, then they can produce chain reactions in the mass that is decaying, sometimes causing a larger effect of ionization.
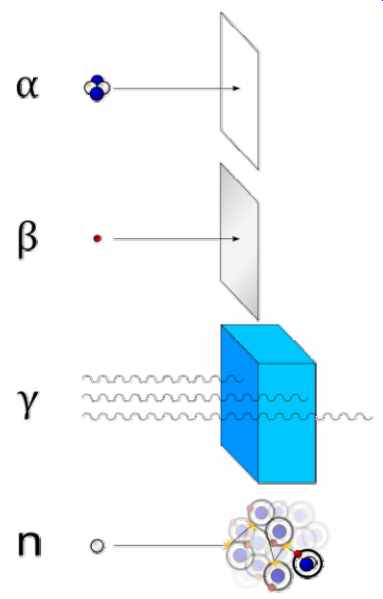
--- Types of radiation: gamma rays are represented by wavy lines, charged particles
and neutrons by straight lines. The little circles show where ionization processes
occur.
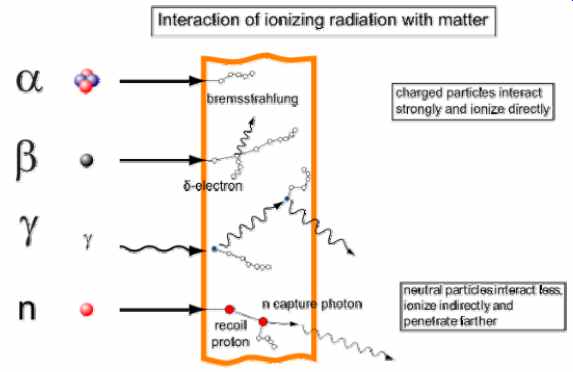
An ionization event normally produces a positive atomic ion and an electron. High energy beta particles may produce bremsstrahlung when passing through matter, or secondary electrons (d-electrons); both can ionize in turn. Energetic Beta-particles. like those emitted by 32 P, are quickly decelerated when passing through matter. The energy lost to deceleration is emitted in the form of X-rays called "Bremsstrahlung" which translates "Braking Radiation". Bremsstrahlung is of concern when shielding beta emitters. The intensity of bremsstrahlung increases with the increase in energy of the electrons or the atomic number of the absorbing medium.
Unlike alpha or beta particles, gamma rays do not ionize all along their path, but rather interact with matter in one of three ways: the photoelectric effect, the Compton effect, and pair production. By way of example, the figure shows Compton effect: two Compton scatterings that happen sequentially. In every scattering event, the gamma ray transfers energy to an electron, and it continues on its path in a different direction and with reduced energy.
In the same figure, the neutron collides with a proton of the target material, and then becomes a fast recoil proton that ionizes in turn. At the end of its path, the neutron is captured by a nucleus in an (n,?)-reaction that leads to a neutron capture photon.
Alpha (a) radiation consists of a fast moving helium-4 (4 He) nucleus and is stopped by a sheet of paper. Beta (ß) radiation, consisting of electrons, is halted by an aluminum plate. Gamma (?) radiation, consisting of energetic photons, is eventually absorbed as it penetrates a dense material. Neutron (n) radiation consists of free neutrons which are blocked using light elements, like hydrogen, which slow and/or capture them.
Various types of ionizing radiation may be produced by radioactive decay, nuclear fission and nuclear fusion, and by particle accelerators and naturally occurring cosmic rays.
Muons and many types of mesons (in particular charged pions) are also ionizing.
In order for a particle to be ionizing, it must both have a high enough energy and interact with the atoms of a target.
Neutron radiation
Neutron radiation is a kind of non-ionizing radiation which consists of free neutrons. A result of nuclear fission or nuclear fusion, it consists of the release of free neutrons from both stable molecules and isotopes, and these free neutrons react with nuclei of other stable molecules to form new isotopes of previously non-isotopic molecules, which in turn produce radiation. This will result in a chain reaction of nuclear radiation, which makes radiation dangerous and harmful over great areas of space.
Sources
Neutrons may be emitted from nuclear fusion or nuclear fission, or from any number of different nuclear reactions such as from radioactive decay or reactions from particle interactions (such as from cosmic rays or particle accelerators). Large neutron sources are rare, and are usually limited to large-sized devices like nuclear reactors or particle accelerators (such as the Spallation Neutron Source). Neutron radiation was discovered as a result of observing a beryllium nucleus reacting with an alpha particle thus transforming into a carbon nucleus and emitting a neutron, Be(a, n)C. The combination of an alpha particle emitter and an isotope with a large (a, n) nuclear reaction probability is still a common neutron source.
Uses
Cold, thermal and hot neutron radiation is most commonly used for scattering and diffraction experiments in order to access the properties and the structure of materials in crystallography, condensed matter physics, biology, solid state chemistry, materials science, geology, mineralogy and related sciences. Neutron radiation is also used in select facilities to treat cancerous tumors due to its highly penetrating and damaging nature to cellular structure. Neutrons can also be used for imaging of industrial parts termed neutron radiography when using film, neutron radioscopy when taking a digital image, such as through image plates, and neutron tomography for three dimensional images.
Neutron imaging is commonly used in the nuclear industry, the space and aerospace industry, as well as the high reliability explosives industry.
Ionization mechanisms and properties
Neutron radiation is often called indirectly ionizing radiation. It does not ionize atoms in the same way that charged particles such as protons and electrons do (exciting an electron), because neutrons have no charge. However, neutron interactions are largely ionizing, for example when neutron absorption results in gamma emission and the gamma subsequently removes an electron from an atom, or a nucleus recoiling from a neutron interaction is ionized and causes more traditional subsequent ionization in other atoms. Because neutrons are uncharged, they are more penetrating than alpha radiation or beta radiation. In some cases they are more penetrating than gamma radiation, which is impeded in materials of high atomic number. In hydrogen, a low-energy neutron may not be as penetrating as a high energy gamma.
Health hazards and protection
In health physics neutron radiation is considered a fourth radiation hazard alongside the other types of radiation. Another, sometimes more severe hazard of neutron radiation, is neutron activation, the ability of neutron radiation to induce radioactivity in most substances it encounters, including the body tissues of the workers themselves. This occurs through the capture of neutrons by atomic nuclei, which are transformed to another nuclide, frequently a radionuclide. This process accounts for much of the radioactive material released by the detonation of a nuclear weapon. It is also a problem in nuclear fission and nuclear fusion installations, as it gradually renders the equipment radioactive; eventually the hardware must be replaced and disposed of as low-level radioactive waste.
Neutron radiation protection relies on radiation shielding. In comparison with conventional ionizing radiation based on photons or charged particles, neutrons are repeatedly bounced and slowed (absorbed) by light nuclei, so a large mass of hydrogen rich material is needed. Neutrons readily pass through most material, but interact enough to cause biological damage. Due to the high kinetic energy of neutrons, this radiation is considered to be the most severe and dangerous radiation available. The most effective materials are e.g. water, polyethylene, paraffin wax, or concrete, where a considerable amount of water molecules are chemically bound to the cement. The light atoms serve to slow down the neutrons by elastic scattering, so they can then be absorbed by nuclear reactions. However, gamma radiation is often produced in such reactions, so additional shielding has to be provided to absorb it.
Because the neutrons that strike the hydrogen nucleus (proton, or deuteron) impart energy to that nucleus, they in turn will break from their chemical bonds and travel a short distance, before stopping. Those protons and deuterons are high linear energy transfer particles, and are in turn stopped by ionization of the material through which they travel.
Consequently, in living tissue, neutrons have a relatively high relative biological effectiveness, and are roughly ten times more effective at causing cancers or LD-50s compared to photon or beta radiation of equivalent radiation exposure.
Effects on materials
Neutrons also degrade materials; bombardment of materials with neutrons creates collision cascades that can produce point defects and dislocations in the materials. At high neutron fluences this can lead to embrittlement of metals and other materials, and to swelling of some of them. This poses a problem for nuclear reactor vessels, and significantly limits their lifetime (which can be somewhat prolonged by controlled annealing of the vessel, reducing the number of the built-up dislocations). Graphite moderator blocks are especially susceptible to this effect, known as Wigner effect, and have to be annealed periodically; the well-known Windscale fire was caused by a mishap during such an annealing operation.
Neutron radiation and nuclear fission
The neutrons in reactors are generally categorized as slow (thermal) neutrons or fast neutrons depending on their energy. Thermal neutrons are similar to a gas in thermodynamic equilibrium but are easily captured by atomic nuclei and are the primary means by which elements undergo atomic transmutation.
In order to achieve an effective fission chain reaction, the neutrons produced during fission must be captured by fissionable nuclei, which then split, releasing more neutrons.
In most fission reactor designs, the nuclear fuel is not sufficiently refined to be able to absorb enough fast neutrons to carry on the fission chain reaction, due to the lower cross section for higher-energy neutrons, so a neutron moderator must be introduced to slow the fast neutrons down to thermal velocities to permit sufficient absorption. Common neutron moderators include graphite, light water and heavy water. A few reactors (fast neutron reactors) and all nuclear weapons rely on fast neutrons. This requires certain changes in the design and in the required nuclear fuel. The element beryllium is particularly useful due to its ability to act as a neutron reflector or lens. This allows smaller quantities of fissile material to be used and is a primary technical development that led to the creation of neutron bombs.
Cosmogenic neutrons
Cosmogenic neutrons, neutrons produced from cosmic radiation in the Earth's atmosphere or surface, and those produced in particle accelerators can be significantly higher energy than those encountered in reactors. Most of them activate a nucleus before reaching the ground; a few react with nuclei in the air. The reactions with nitrogen 14 lead to the formation of carbon 14, widely used in radiocarbon dating.
SILC (semiconductors)
Stress Induced Leakage Current (SILC) is an increase in the gate leakage current of a MOSFET, due to defects created in the gate oxide during electrical stressing. SILC is perhaps the largest factor inhibiting device miniturization. Increased leakage is a common failure mode of electronic devices.
Oxide Defects
The most well studied defects assisting in the leakage current are those produced by charge trapping in the oxide. This model provides a point of attack and has stimulated researchers to develop methods to decrease the rate of charge trapping by mechanisms such as N2O nitridation of the oxide.
Hot carriers injection
Hot carriers injection (HCI) is the phenomenon in solid-state or semiconductor electronic devices where either an electron or a "hole" gains sufficient kinetic energy to overcome a potential barrier necessary to break an interface state. The term hot electron comes from the effective temperature used to model carrier density (with fermi dirac function). One should not think that the term "hot" refers to the actual temperature of the MOS transistor. At higher temperatures, the mean free path (distance between two collisions with atoms in the substrate) is shorter, which decreases the energy gain by the carrier. As a result, Hot Carrier Degradation is more important at low temperature.
HCI and CMOS Semiconductor Technology
Semiconductor Physics of HCI
The term "hot carrier injection" usually refers to the effect in MOSFETs, where a carrier is injected from the conducting channel in the silicon substrate to the gate dielectric, which usually is made of silicon dioxide (SiO2). To become "hot" and enter the conduction band of SiO2, an electron must gain a kinetic energy of 3.3 eV. For holes, the valence band offset in this case dictates they must have a kinetic energy of 4.6 eV. When electrons are accelerated in the channel, they gain energy along the mean free path.
This energy is lost in two different ways:
1. The carrier hit an atom in the substrate. Then the collision create a cold carrier and an additional electron-hole pair. In the case of nMOS transistors, additional electrons are collected by the channel and additional holes are evacuated by the substrate.
2. The carrier hit a Si-H bond and break the bond. An interface state is created and the Hydrogen atom is released in the substrate.
The probability to hit either an atom or a Si-H bond is random. And the average energy involved in each process is the same in both case.
This is the reason why the substrate current is monitored during HCI stress. A high substrate current means a large number of created electron-hole pairs and thus an efficient Si-H bond breakage mechanism.
When interface states are created, the threshold voltage is modified and the subthreshold slope is degraded. This lead to lower current, and degrade the operating frequency of integrated circuit.
Scaling and HCI
Advances in semiconductor manufacturing techniques and ever increasing demand for faster and more complex integrated circuits (ICs) have driven the associated Metal- Oxide-Semiconductor field-effect transistor (MOSFET) to scale to smaller dimensions.
However, it has not been possible to scale the supply voltage used to operate these ICs proportionately due to factors such as compatibility with previous generation circuits, noise margin, power and delay requirements, and non-scaling of threshold voltage, subthreshold slope, and parasitic capacitance.
As a result internal electric fields increase in aggressively scaled MOSFETs, which comes with the additional benefit of increased carrier velocities (up to velocity saturation), and hence increased switching speed, but also presents a major reliability problem for the long term operation of these devices, as high fields induce hot carrier injection which affects device reliability.
Large electric fields in MOSFETs imply the presence of high-energy carriers, referred to as "hot carriers". These hot carriers that have sufficiently high energies and momenta to allow them to be injected from the semiconductor into the surrounding dielectric films such as the gate and sidewall oxides as well as the buried oxide in the case of silicon on insulator (SOI) MOSFETs.
CMOS Reliability Impact of HCI
The presence of such mobile carriers in the oxides triggers numerous physical damage processes that can drastically change the device characteristics over prolonged periods.
The accumulation of damage can eventually cause the circuit to fail as key parameters such as threshold voltage shift due to such damage. The accumulation of damage resulting degradation in device behavior due to hot carrier injection is called "hot carrier degradation". The useful life-time of circuits and integrated circuits based on such a MOS device are thus affected by the life-time of the MOS device itself. To assure that integrated circuits manufactured with minimal geometry devices will not have their useful life impaired, the life-time of the component MOS devices must have their HCI degradation well understood. Failure to accurately characterize HCI life-time effects can ultimately affect business costs such as warranty and support costs and impact marketing and sales promises for a foundry or IC manufacturer.
Relationship to Radiation Effects
Hot carrier degradation is fundamentally same as the ionization radiation effect known as the total dose damage to semiconductors, as experienced in space systems due to solar proton, electron, X-ray and gamma-ray exposure.
HCI and NOR Flash Memory Cells
HCI is the basis of operation for a number of non-volatile memory technologies such as Electrically Erasable Programmable Read-Only Memory (EEPROM) cells. As soon as the potential detrimental influence of HC injection on the circuit reliability was recognized, several fabrication strategies were devised to reduce it without compromising the circuit performance.
NOR flash memory exploits the principle of hot carriers injection by deliberately injecting carriers across the gate oxide to charge the floating gate. This charge alters the MOS transistor threshold voltage to represent a logic '0' state. An uncharged floating gate represents a '1' state. Erasing the NOR Flash memory cell removes stored charge through the process of Fowler-Nordheim tunneling.
Because of the damage to the oxide caused by normal NOR Flash operation, HCI damage is one of the factors that cause the number of write-erase cycles to be limited. Because the ability to hold charge and the formation of damage traps in the oxide affects the ability to have distinct '1' and '0' charge states, HCI damage results in the closing of the non-volatile memory logic margin window over time. The number of write-erase cycles at which '1' and '0' can no longer be distinguished defines the endurance of a non-volatile memory.