AMAZON multi-meters discounts AMAZON oscilloscope discounts
1. Glass
1.1 Introduction
When one talks of chemistry (in general) or working in a laboratory (in specific), the average person usually envisions an array of glassware-test tubes, beakers, coiled tubes, and the like. This list obviously is not the sum and substance of laboratory equipment (or chemistry for that matter), but items made of glass will always play a predominant role in most laboratories.
Glass is used in the laboratory because it has three important properties. First, it is transparent and thus allows the user to observe a reaction taking place within a container. Second, it is very stable and is nonreactive to many materials used in the laboratory. Third, it is (relatively) easily malleable, allowing new designs and shapes of apparatus to be created, produced, and, if broken, repaired.
The nature of glass, its structure, and its chemistry, as well as the shapes and designs made from it, have come from years of development. The standard shapes of the beaker, Erlenmeyer flask, and round-bottom flask were each developed to meet specific needs and functions. Variations in wall thicknesses, angles, and height-to-width ratios are all critical for specific functions of each container.
Before discussing any container, however, it may be best to talk of the containment material: what it is, what makes it unique, and what are its strengths and weaknesses. We can then relate these properties to specific types of containers and know why they are made as they are. By understanding the shape, design, and function of laboratory glassware, one can achieve greater efficiency and proficiency in the laboratory.
1.2 Structural Properties of Glass
The standard definition of glass is a "non-crystalline solid." In 1985, the ASTM (American Society for Testing and Materials) referred to glass as "... an inorganic product of fusion that has cooled to a rigid state without crystallizing." However, since 1985, glasses of organic and metallic materials have been produced. Therefore, a more accurate definition of a glass would be "any material that is cooled fast enough to prevent the development of crystals." Any further reference to the term "glass" in this guide refers specifically to the inorganic glasses that we normally associate as glass. As we shall see, this specific type of glass has properties that facilitate the creation of the "glass state."
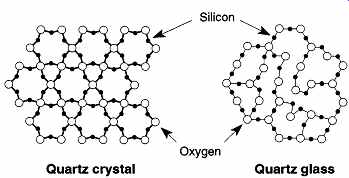
FIG. 1 The molecular structure of a quartz
crystal and quartz glass.
The key structural concept of glass, is that it is not a crystal. This concept holds true for high-temperature quartz glass and for ornate lead crystal glass. A crystal, by definition, is a collection of atoms in a repeating sequence and form that develops or has symmetry in its structure. By comparison, the structure of glass at the atomic level is a three-dimensional web of interconnecting oxides of materials, frozen in place by rapid cooling. Thus, glass made of inorganic materials may be defined as a super-cooled liquid composed of a mixture of oxides in a solution.
To better understand what glass is, and why different glasses have different properties, one should first compare crystals and glass at the molecular level. This comparison begins with the molecular structure of a quartz crystal. The quartz crystal is composed of (essentially) pure SiO2, the same molecular composition of quartz glass, and the chief component material of most glass. The quartz crystal is a silicon tetrahedra composed of one atom of silicon surrounded by four oxygen atoms, in a tight three-dimensional network of high-energy bonds. The extra two oxygen atoms, bridging oxygens, interconnect the silicon atoms into a three dimensional network. The left side of FIG. 1 illustrates a two-dimensional representation of one plane of such a network.
If you take a quartz crystal, heat it until it melts, and allow it to rapidly cool, the even network of rings is broken into nonuniform irregular chains (see right side of FIG. 1). These glass chains have no discrete crystalline form as they interconnect throughout the entire glass item. The cooled SiO2 is now called either "fused quartz," "fused silica", or "quartz glass." The bonds between the Si and O atoms are still high-energy bonds, and tremendous amounts of thermal energy are still required to disturb and break those bonds. One can also see from FIG. 1 that the interstice regions in the glass form of SiO is much larger than the crystal form.
The effects of the new molecular arrangement are particularly apparent under the influence of varying temperature and gas permeability.
When another oxide molecule replaces a silicon oxide tetrahedron, the connecting oxygen atom is considered a non-bridging oxygen. The inclusion of these materials breaks up the continuity of the structural network and provides the glass with significant changes in properties. Thus, the uniformity of the bonds within this glass web does not exist as it once did with pure SiO2. Therefore, significantly less energy is required to break the bonds than with pure SiO2 glass, and less heat is required to melt the new glass. In addition, all properties of the glass changes as the composition of the glass changes. These changes include their optical, thermal, and expansion properties. By changing the raw materials of a glass, various properties can be enhanced or suppressed by a manufacturer to meet very exacting requirements.
When a crystal breaks, it tends to fracture on a cleavage plane based on its molecular structure. That is why a large table salt crystal will break into fragments that maintain the original geometric shape and surface angles of the original crystal. When glass is broken, only amorphous, irregular shapes remain, because glass has no structural geometric consistency. This lack of structural consistency occurs in all glasses, and it is impossible to distinguish one type of glass from another based on a fracture pattern.* Another difference between a glass and a crystal is that if you take (for example) a cubic crystal of table salt and heat it until it melts, the crystal cube will start to slump into a puddle at a specific temperature (801°C). The melting and/or freezing temperature of table salt, as with most materials, is usually considered the point at which the solid and liquid form of the material can exist together.
Glass, on the other hand, has no single fixed melting point. It maintains its physical shape after it begins to soften. External forces such as gravity will cause the glass to sag under its own weight once temperatures are above the softening point.
Gradually as the temperature continues to rise, the surface will begin to lose form.
Then, internal forces, such as surface tension, cause sharp corners on the glass to round as the glass "beads up" on itself. Eventually, higher temperatures will cause the glass to collect into a thick, liquid puddle. The term "liquid" here is rather nebulous, because the viscosity of glass is like honey.
The hotter a glass "liquid" gets, the less viscous it becomes. It is, in fact, the high viscosity of this puddle that helps prevent glass from crystallizing: As glass cools past the crystallization temperature, its high viscosity inhibits atomic mobility, preventing the atoms from aligning themselves into a crystalline form.
[
Because glass fracture patterns are consistent throughout all glass types, these markings can help provide clues to the origin and cause of glass fracture.
Quartz glass never achieves this liquid, "honey-like" state.
]
It has already been mentioned that glass does not have a specific melting temperature. Rather, its viscosity gradually changes as the temperature varies. The viscosity decreases until the glass is identified as being in a melted state. Thus, glass scientists define important transitional changes based on specific viscosity ranges of the glass in question. There are four significant viscosities of glass (in comparison to the viscosity [poises] numbers presented below, the viscosity of glass at room temperature is 10^22+ poises):
1. Strain Point (~10^14.5 poises): Anything above this temperature may cause strain in glass. Internal stresses may be relieved if the glass is baked in an oven for several hours at this temperature
2. Annealing Point (=10^13 poises): If an entire item were uniformly baked at this temperature, the item would be relieved of strain in about 15 minutes. The annealing range is considered to be the range of temperatures between the strain point and the annealing point.
3. Softening Point (=10^7.6 poises) Glass will sag under its own weight at this temperature. The surface of the glass is tacky enough to stick (but not fuse*) to other glass. The specific viscosity for the softening point depends on both the density and surface tension of the glass.
4. Working Point (=10^4 poises) Glass is a very thick liquid at this point (like honey) and can be worked by most conventional glassblowing techniques. At the upper end of the working range, glass can be readily fused together or worked (if the glass is heated too high, it will boil and develop characteristics that are different from those of the original glass). At the lower end of the working range, glass begins to hold its formed shape.
The fact that glass is a solid at room temperatures should not be underestimated, and the statement "solids cannot flow" cannot be over emphasized. There is a romantic notion that windows in old churches in Europe (or old colonial homes in the United States) have sagged over time. The common belief that the glass is thicker on the bottom than on the top because of such sagging is incorrect. Studies by F.M. Emsberger, an authority on viscoelastic behavior of glass, showed that glass will not permanently sag under its own weight at room temperature.
[Metals can be welded or stuck together so that they cannot be separated. Glass, on the other hand, must be fused by heating two separate pieces of glass to the point at which they flow together and become one piece. If glass pieces are simply "stuck" together, it is relatively simple to break them apart.
This amount of stress without fracture was accomplished by covering just newly made glass with lacquer, which prevented surface flaws and surface hydration. Normally, glass cannot receive this amount of stress without breaking.]
Ernsberger took several 1/4" glass rods and bent them 1.7 cm off center over a 20-cm span. The amount of bending stress was calculated to be about 150,000 lb/ in.^2 significantly greater than any stress received from simple sagging. After 26 years, one of the rods was released from the strain: within 48 hours it returned to its original shape.
R.C. Plumb offers an excellent theory as to why old windows are sometimes thicker on the bottom than on top. He reports that the old technique of manufacturing windows involved collecting a large amount of melted glass at the end of a metal blowpipe, blowing a vase, and attaching the vase bottom to a solid metal rod called a ponty. The end that was blown into is now removed, leaving an open end pointing away from the glassblower. By reheating and then rapidly spinning the hot (soft) vase, the glassblower would use centrifugal force to make the open end flair out, thus transforming the vase into a flat circular pane up to five feet in diameter. From this pane (or "table," as it was called), the glassblower would cut square sections. The sections would have varying thicknesses depending on how far from the center of the "table" they were cut.
Plumb does not offer a strong reason as to why he believes the thicker sections were placed on the bottom. He states, "It would certainly make good sense to install the glass with the thick edge down!" I am unaware of anyone acknowledging if any windows have ever been found to be thicker on the top than on the bottom. It is conceivable that if any such windows were ever noticed, they were disregarded because they did not fit into the pre-expected pattern of being thicker on the bottom.
Because of the belief that glass may sag under its own weight, there has been concern about the storage of glass tubing and rods at an angle. The only danger to glass being stored at an angle is fear of damage to the ends of the glass. Other wise, there is no problem with storing glass vertically, at an angle, or on its side.
1.3 Phase Separation
Pure quartz glass, lead glass, borosilicate glass, or any other type of glass that is clear is in phase. In-phase glass is completely homogeneous throughout. Glass that has any cloudy nature to its appearance can easily be discerned as being out of phase or has phase separation. The cloudy nature is due to inseparable phase (or materials) from the glass phase. As mentioned, glass is glass because it cools too fast for the molecules to align themselves into their crystalline structure. If there are nucleating agents that can enhance the growth of crystals or if the glass is held at too hot a temperature for too long, some crystallization will occur.
Sometimes phase separation can be visually desirable such as that which occurs in opaline glass. By placing an earth alkali fluoride or phosphate material on the surface of the glass, the quickly generated fine-crystalline surface disperses light so efficiently that an opal glow is created. Photosensitive glass is an excellent example of a more practical/commercial use of phase separation. This phase separation is activated by ultraviolet light; and once the ultraviolet light is removed, the glass re-phases to the glassy state.
Phase separation is not always a surface phenomenon. The glass that is eventually changed into a pyroceramic material has a nucleating agent mixed throughout the original vitreous material. After the object has been formed and examined, it is slowly baked through its phase separation in an oven.
Vycor®, a high-temperature glass that often can be substituted for quartz glass, is also made by a phase separation process.
The phase separation producing opalescence and photosensitivity are production-created. That is, during the production of the glass, the phase separation occurs. The phase separation that occurs with pyroceramic material and Vycor requires baking the glass at high temperatures for an extended time. This elevated temperature provides the time for the molecules to align and/or separate them selves in a crystalline pattern.
Unfortunately, not all phase separation is desirable. When borosilicate glass is heated for too long near its annealing temperature, a phase separation will occur.
This tends to exhibit itself throughout the glass, but can only be observed with an electron microscope. Despite it not being observable to the naked eye, the ramifications of this separation are considerable. The glass separates itself into two phases: One is rich in silicic acid, while the other is rich in alkali borate.
The result of this change is that the glass has much greater sensitivity to chemical attack.
The significance of the chemical attack sensitivity can best be demonstrated by heat exchangers that must deal with high-temperature water. There are several issues and conditions that come together for this effect:
1. Heat exchangers are made of thick glass.
Because thick glass requires a longer annealing process, there is a greater opportunity for phase separation to occur.
2. Due to the manufacturing process, they must go through the annealing process several times.
Phase separation is a result of the total length of time the glass is held to high temperatures, not the length of time at any one setting.
3. Glasses with high alkali content are more susceptible to chemical attack.
Water is not generally thought of as a caustic material, but it can be to less chemically resistant glass (e.g., lead and soda-lime glass). Even soda-lime glass that has too great a percentage of soda is more chemically vulnerable than a soda-lime glass with a lower percentage of soda.
Generally, borosilicate glass is generally very resistant to water. How ever, if the alkali concentration is too high (due to phase separation) and this glass is subjected to high-temperature water (more corrosive than room temperature water), greater glass erosion can be expected.
Because the thick glass (of a heat exchanger) that had been annealed several times is now confronting hot water, it is more likely to fail (corrode and break) than other borosilicate glassware.
Aside from being initiated by sitting in hot ovens for too long a period of time, phase separation can also occur when a glass is worked too long or too often. This is why glass can only be repaired a limited number of times. After too many repairs, glass devitrifies (or re-crystallizes, a symptom of phase separation) while being worked (see next section), and this de-vitrification does not disappear by heating. There are five items to consider for limiting the possibility, or degree of phase separation due to annealing operations.
1. All annealing procedures to which an article is subjected before completion must be added together.
2. The number of annealing steps should be kept as small as possible
3. Since the level of the annealing temperature and the duration of the annealing method tend in the same direction (i.e., phase separation), these should be limited whenever possible.
4. The annealing temperature should not exceed 550°C.
5. Each separate annealing period should not exceed 30 minutes. Should an article have to be annealed several times, the sum of all annealing periods should not exceed two hours.
Regardless of the heating processes, phase separation will occur if the glass was not cleaned prior to annealing. Salts (from finger prints), silicone grease, water spots, and other contamination can "burn into" the glass, creating nucleation points from which phase separation will originate.
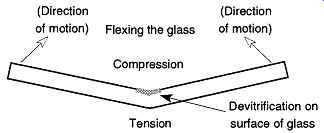
FIG. 2 Creating devitrification in glass.
[Extremely hot water, by its very nature, is significantly more corrosive than room temperature water which compounds the problem.]
1.4 Devitrification
Devitrification is the recrystallization of glass. Glass that is devitrified appears frosty (translucent) and is no longer transparent. Devitrified glass is structurally weaker and is more vulnerable to chemical attack. If a glass is held within its crystallization temperature* for a sufficiently long time, phase separation occurs and the atoms have time to align themselves into a crystalline structure. Once the temperature is allowed to drop, the glass becomes increasingly more viscous, until it cannot further devitrify.
There are several ways to force glass to devitrify (whether devitrification is desired or not). One technique is to heat the glass until it begins to soften, then mechanically work, or flex, the glass while it cools. Eventually a whitish frost will appear on the surface in the region of compression (see FIG. 2).
The risk of devitrification rises the longer a glass is kept in a softened or melted state, and it is also linked to how dirty the glass is. Devitrification typically begins as a surface phenomenon, using either dirt or some other surface defect as a nucleation point.
The devitrification process may be assisted by variations in the exterior composition (which is typically different from the interior) of a glass object.
These variations may be the result of flame-working the glass, surface contamination, or chemical attack.
Devitrification can often be removed by reheating a glass up to its melting temperature and avoiding any mechanical action while it re-cools to a rigid state. How ever, if a glass is overworked or dirty when originally flame-heated, removal of the devitrification may be impossible. If glass has been held too long at an annealing temperature or has been repaired too often, it may not be possible to remove the devitrification.
Mechanical stress is not a requirement for devitrification. The phenomenon is also common in quartz glass furnace tubes maintained at high temperatures for extended periods of time. Devitrification of silica occurs at increasing rates from 1000°C to 1710°C, which is the crystallization temperature and melting point range of B-cristobalite.
Insufficient surface cleaning and very slow cool-down times typically facilitate devitrification on these tubes. Early-stage devitrification on a quartz glass furnace tube may be removed by a hydrofluoric acid dip. This cleaning procedure can remove only surface cristobalite. Devitrification deeper than surface level cannot be removed.
[The specific crystallization temperature is not commonly identified, but typically is between the annealing and softening points.
Cristobalite is transparent. We do not normally consider devitrified glass as a transparent material. However, once fused silica has cooled below 250°C, 6-cristobalite is transformed into a-cristobalite. This substance is the white opaque material we usually associate with devitrified silica. When fused silica is reheated into the devitrification range, the a-cristobalite turns back into 6-cristobalite.
However, because a-cristobalite has many fissures and cracks, the opacity remains when it is reheated back into fi-cristobalite.] The best way to limit or prevent devitrification on quartz glass is to ensure that it is maintained scrupulously clean: No fingerprints, oils, dirt, or chemicals of any kind should get on the surface. Also, temperatures above 1200°C should be limited as much as possible.
Note that devitrification is a nucleation process. That is, devitrification does not confine itself within the area where it begins. Rather, once started, it spreads like mold on a piece of fruit. Thus, a drop of tap water, a fingerprint, or any other localized contaminant can initiate devitrification, which can then spread over the entire surface of the container.
Quartz is not the only glass that is prone to premature destruction due to devitrification. Aluminosilicate glass used on halogen lamp bulbs are also prone to this problem. Handling halogen lamp bulbs with bare fingers can significantly decrease their operation life by depositing fingerprint oils on the surface.
To maximize the life of halogen lamp bulbs, they should only be handled with cot ton cloves. Although cleaning the bulbs after handling will help, it is best not to handle them at all.
1.5 Different Types of Glass Used in the Lab
Different properties of glass can be exploited by combining different oxides in a glass mix. There are thousands of different commercially made glasses (although many of these glasses overlap in type and characteristics). The glass used in a lab oratory can be divided into three major categories: soft glasses, hard glasses, and high-temperature and UV-transmission glasses. Table 2 in the next section lists selected properties of these various glasses.
Among the properties of glass that are of particular interest to glass scientists are its expansion properties (the amount of size increase in relation to temperatures increases, refractive index (how light passes through the glass), viscosity (the consistency of the glass as temperature increases), and its dielectric proper ties (how electrically resistant the glass is at various temperatures).
One of the problems with glass composition and its relations to its properties is that many of the properties change depending on its temperature. For example, as glass gets hotter, it becomes less electrically resistive while at the same time permeation rates increase. Thus, one may need to consider the temperature the glass will be used in while selecting a glass for specific purposes.
When creating a glass, one has to watch all the properties. This is because as you increase one material to obtain certain desirable properties, other properties are likely to change which may or may not be desirable. For example, by adding PbO to silicate glass, there is an increase in viscosity while at the same time there is a decrease in electrical resistivity.
This section occasionally lists the contents of glass types. The glass industry typically lists the component chemicals of glass in their oxide states as percent ages of total weight. Chemically speaking, this practice does not make sense because when you add CaO and Na2O to SiO2, you are actually adding Ca + 2Na + Si + 4O. Chemists do not consider this industrial approach proper because it does not look at the reaction from a proper chemical perspective. Regardless, I shall defer to the industry standard simply because it is easier to explain variations in glass properties according to changes in concentration percentages. In addition, this convention is used because these materials must be added to a glass melt in their oxide states or they will not "glass." Please note: Glasses of different compositions should never be mixed together for recycling. Therefore, you should never take a box of used and/or broken glass ware from the lab to the local recycling yard. For one thing, you risk contamination by toxic materials left on inadequately washed broken glassware (which could violate environmental laws). Equally important, if glass of the wrong type should be mixed with commercially recyclable types of glass, an entire glass melt would be wasted. From an environmental standpoint, the best thing you can do in the lab is to avoid waste and carelessness. Damaged glassware should be discarded in a proper receptacle. Final disposal of glassware depends on its history and contaminants. For example, nontoxic materials can be sent directly to a land fill, but bio-organic residue may need to be sterilized before disposal. Glassware contaminated with heavy metals or radioactivity may require disposal at an appropriate hazardous waste disposal site.
The Soft Glasses. The soft glasses receive their name identification by the fact that they are physically softer than other glasses; that is, they abrade more easily.
In addition, these glasses tend to maintain their soft working properties over a greater temperature range. Thus, they remain "soft," or workable, longer than hard glasses.
Commercially soft glass is used in items such as window panes, bottles, jars, and drinking glasses. Soft glasses can take colors readily (as brown and green bottles show and as do the many hues and colors that are created by artisans such as the Italian glass masters).
[Often, the colors you see on glass animals (made out of borosilicate glass) are acrylic paint, but there are now borosilicate glasses that are colored and readily available.]
The most common soft glass is soda-lime glass. The soda is a carbonate of sodium or sodium oxide (Na2O), and the lime is calcium oxide (CaO) or magnesium oxide (MgO). The materials for this glass are relatively inexpensive, and because of its lower energy demands (lower melting temperatures) and long working times, it is the most inexpensive to manufacture. This glass is also easily recycled.
Not all soft glasses are of equal quality: some are significantly inferior to others.
The quality of a soft glass may be based on the proportions of its constituent materials. Typically, lime is present in concentrations of 8% to 12% (by weight) and soda in concentrations of 12% to 17% (by weight).
If lime concentration levels are too high, devitrification can occur during the manufacturing process.
Conversely, if the lime is held too low (or the alkali concentration is too high), the glass is subject to easy weathering and attack by water. Thus, drinking glasses that spot easily and are difficult to clean may be the result of a low lime or high alkalinity concentration glass rather than bad soap or hard water.
An extreme example of a glass that can be easily weathered is sodium silicate, also known as soluble silicate or, informally, waterglass. The sodium content in sodium silicate is so high that the glass can be dissolved in water and shipped as a liquid glass. Sodium silicate is used as an adhesive, cleaner, and protective coating material.
Sodium silicate is stored and shipped as a liquid, but when exposed to air, it will turn into a hard, glass-like material. In reality, it is a glass in a totally anhydrous form; the sodium hydroxide content is 34% by weight, and the remaining 66% is silicon dioxide. These proportions are theoretical, however, as an anhydrous sodium silicate is not really possible because it would always be absorbing water from the air.
When sodium silicate is shipped, its contents are typically
-27% SiO2
-14 % NaOH
=59% H2O
These proportions are all approximate because the water percentage can vary significantly. Once the water has evaporated from the sodium silicate, there is still a high level of water left behind. The dried sodium silicate will have the following approximate contents (by weight), although the actual percentages will again vary depending on the atmospheric water:
-49% SiO2
-26% NaOH
-25% H2O
Note that although dry soda content would normally be listed as Na2O, in sodium silicate all the soda is hydrated and, therefore, is identified in the form NaOH.
Many disposable glass laboratory items are made of soft glass. If a laboratory glass item does not specifically bear one of the words Pyrex®, Kimax®, or Duran® in printed or raised letters, it is likely to be a soda-lime type of glass.
Beginning chemistry students usually receive their first glassblowing lesson using soft glass tubes over a Bunsen burner to make bends or eye droppers, because only soft glass is malleable at Bunsen burner temperatures. Thus, if you have a glass tube soft enough to bend over a Bunsen burner, it is a soft glass. Currently, only Wheaton Glass and Schott glass make soda-lime tubing. Wheaton's glass can be purchased from either Wheaton or Friedrich & Dimmock. Schott's soft glass (AR) can be purchased from Schott or from Glass Warehouse.
The other common soft glass is lead glass. Commercially, we see this glass in lead crystal glass, where 22-25% (by weight) of the component material is PbO2.
Although misnamed as a crystal, lead crystal glass nevertheless has beautiful optical properties that are often associated with crystals. The high refractive index of lead glass (one of the highest refractive indexes of all glasses) is the foundation for its effects with light. The other important property of lead glass is its high electrical resistivity. A typical lead oxide glass may be 100 times more electrically resistive than a soda lime glass. A side property of lead crystal glass is its resonance: Decorative bells of lead glass have a beautiful chime, while those of boro-silicate glass often have a dull, dead "clank." One additional application in the laboratory for lead glass is as a shield to protect workers from the harmful rays produced by x-ray machines. For example, the glass window behind which an x-ray technician stands is made of lead glass.
These windows can be made of up to 75% PbO2. This greater quantity of lead oxide in this glass makes the glass three times heavier (6.0 gm/m3 ) than standard laboratory borosilicate glass (2.1 gm/cm^3 ).
Lead glass is very vulnerable to impact abrasion (it is almost twice as soft as soda-lime) and can be extremely sensitive to temperature changes (temperature induced failure is common in lead glass). On the other hand, as previously mentioned, lead glass is among the most electrically resistive glasses available.
Because of that property, lead glass is one of the chief glasses used in electrical components. Neon signs and TV tubes are typically made of lead glass. (Borosilicate glass is being used more and more often for neon work.) Another term for lead (or lead-potash-silica) glass is flint glass. This name can be confusing, because the term has two other meanings in the glass industry: It is used by the container industry to connote colorless glass. The term flint glass is also used by the optical glass industry to connote glass that has a high refractive index and dispersion* of optical rays. (Soda-lime [or potash-lime-silica] glass has a low refractive index and dispersion of optical rays. It is called crown glass.) In addition to soda-lime and lead glass, there are several "specialty" soft glasses in the laboratory. One of the more notable is Exax® made by the Kimble Corporation. This glass repels a static charge, making it particularly useful for holding or weighing powders.
[The dispersion of glass is related to its index of refraction and is based on an analysis of the pas sage through the glass of a yellow helium line (587.6 nm), blue and red hydrogen lines (486.1 and 656.3 nm, respectively), and green mercury line (546.1 nm).]
The biggest drawback with all soft glasses is that any apparatus made with these glass types are essentially non-repairable. Because of their relatively high thermal coefficients of expansion, they are likely to shatter when the flame of a gas-oxygen torch touches them. Additionally, replacement soft glass components such as stopcocks and joints are generally not easily available. Thus, unless an item has particularly unique qualities or value, the cost of component repair is far greater than wholesale replacement. Although soft glass items may be less expensive initially, their long-term costs can be much greater than the more expensive (but less expensively repaired) borosilicate ware.
The Hard Glasses. Borosilicate glass, the most common glass found in the laboratory, is one example of a hard glass. It is considered a hard glass for two reasons. First, its ability to resist impact abrasion is over three times the level of soft glass. Second, it sets at a higher temperature and thus, gets "harder" faster.
(This second quality was a physical characteristic that scientific glassblowers found particularly challenging in the early days of borosilicate glass.) Finally, because the hard glasses have much lower thermal coefficients of expansion than do the soft glasses (see Table 2 to see the thermal coefficients of expansion for various glasses), they can withstand much greater thermal shocks than soft glasses. The hard glasses are also more chemically resistant to alkaline solutions and many other chemicals.
Commercially, borosilicate glasses are found in many consumer products. In the kitchen, oven windows, baking containers, and cooking pot lids all take advantage of the thermal strength of borosilicate glass. In addition, measuring cups are also made out of borosilicate glass, not only for their thermal abilities (like pouring boiling water into a cold measuring cup), but also for their ability to withstand abrasion and impact. Measuring cups are typically nested (smaller cups placed within the bigger cups) and banged around in cupboards and drawers. Borosilicate glass is also used for automobile headlights, as well as for floodlights used in indoor/outdoor lighting.
There is more than one brand and type of borosilicate glass. Pyrex® (by Corning), Kimax® (by Kimble), and Duran® (by Schott) are all brand names of particular borosilicate glasses of similar composition made by different companies.
1 The term "Pyrex" is to borosilicate glass as "Xerox" is to photocopying equipment: It is an almost generic term. There is little difference between the products of these three major companies as far as the chemical makeup of the glass is concerned; in theory, they are all interchangeable. What is important to the user, how ever, is the quality of the glass itself and the quality and uniformity of its manufacturing.
[Any glass containing 5% or more (by weight) of B2O3 (boron oxide) is considered a borosilicate glass.
Also, not all borosilicate glass is the same chemical mix as these glasses. The borosilicate glass used in pipettes is designed for greater chemical resistivity and cannot be sealed to other laboratory ware such as a beaker.]
Borosilicate glass is chemically more resistant than soft glasses and will there fore resist the weathering effects of standing water better than soft glasses.
Although they are resistant to most chemicals, hard glasses are still susceptible to damage from hydrofluoric acid, hot phosphoric acid, and strong alkali solutions.
You should never store any of these solutions in a glass container. A weak alkali solution should not be left for any extended time.
Although the susceptibility of a glass container to chemical attack is a common consideration, there is another issue which is seldom raised: What effect does the consumed glass from a container have on the solution inside the container?
Smith studied the effects of a borosilicate glass container (an Erlenmeyer flask) on sodium and potassium hydroxide solutions of varying molarities at both room temperature and boiling point.
The percentage of molarity change was greatest (approximately a -17% change)* when a low-molarity solution of 0.001148 M aqueous NaOH was boiled. Higher molarity solutions (0.1116 M aqueous NaOH) still provided an impressive change when boiled (approximately -3%). Changes were even evident (approximately -0.02%) when the solution (0.1029 M aqueous NaOH) was maintained at room temperature.
Other common borosilicate glasses are used on pipettes. These borosilicate glasses are more chemically inert than common laboratory borosilicate glass.
Their different composition makes them unable to be fused directly to common laboratory borosilicate glass; therefore, they require use of what is called grading glass between this glass and other borosilicate glasses.
One special category of hard glasses is the aluminosilicate glasses. They are made by the addition of alumina (aluminum oxide) to the glass mix. Aluminosilicate glasses are used in high temperature lamps because they maintain higher viscosities at higher temperatures. Thus, they maintain their shapes at temperatures that would cause borosilicate glass to sag. Aluminosilicate glasses are principle components in many ceramics and fiberglass, and (along with the lead glasses) they are used in many electronic applications. In addition, aluminosilicate glasses are used in mass spectrometers, atomic clocks, and magnetometers. Aluminosilicate glass is also used extensively in halogen bulbs.
The other main attribute of aluminosilicate glass is it's ability to contain helium.
Most glass containers cannot contain helium for extended periods of time, because the small atoms leak past the glass' molecular network. Aluminosilicate glass is considered helium leakproof and is used to contain helium for long periods in a laboratory glass system.
[The approximate percent change is used because four tests were presented for each value. I have presented average figures for these tests.
Kohl points out that the diffusion past quartz glass is 10 million times greater than quartz crystal.]
To appreciate the differences that different compositions of glass can effect the diffusion of helium through glass, Souza interpolated the data of Altemose into actual time. Across a given glass thickness, helium would take two months to cross quartz glass; two years to cross Pyrex, and 20,000 years to cross 1720 glass.
It would also take some 1.6 million years to cross quartz crystal/ but since quartz crystal can't be manipulated to other shapes without changing it into quartz glass, this advantage would be lost.
Aluminosilicate glass is very difficult to work with because it is very prone to re-boil. That is, while bringing it a working temperature, the glass develops bubbles over the surface that are impossible to remove. One other challenge with aluminosilicate glass, which is different from other glasses, is that you can't clean it with hydrofluoric acid. If attempted, it will cause the surface to develop a translucent sheen that is also impossible to remove. It is safe (for the glass) to clean the surface with nitric acid.
The High-Temperature and UV-Transmission Glasses. The last types of glass found in the laboratory are the quartz and UV-transmission glasses. From the many names that are used to describe this type of material, there may be con fusion as to what to call it. It is often just called quartz, but this name can be deceiving because the term quartz could equally refer to the mineral quartz (which is a crystal) or amorphous silica (which is a glass).
Historically, fused quartz referred to transparent products produced from quartz crystal rock, and fused silica referred to opaque products produced from sand.
With the advent of new manufacturing techniques, transparent products can now be produced from sand, so the old distinction is no longer applicable. Currently, the term fused quartz is used whenever the raw product is either quartz rock or sand. The term fused silica is used whenever the raw product is synthetically derived (from SiCl4). Generically, the term quartz glass or, better yet, vitreous sil ica can be used to cover the whole range of materials.
The average consumer is only likely to come across vitreous silica when purchasing special high-intensity, high-brightness light bulbs for specialized lamps such as stadium lights, flash lamps (for cameras), or stroboscopes. Because of its purity, vitreous silica is essential in the manufacture of many consumer products that would otherwise be impossible to manufacture. One such product is the ubiquitous silicon chip that controls everything from computers and calculators to toys and cars. To maintain the purity of chips during construction, the silicon wafers are baked in large tubes of ultrahigh-purity fused silica.
[Silica, or SiO2, occurs in nature as quartz, cristobalite, or tidymite.
Fused quartz is likely to have sufficiently high impurity levels to jeopardize the purity (and thereby the quality) of the final product.]
Whereas other glasses obtain their unique properties by the addition of various oxides, the lack of other materials provides quartz glasses with their unique properties. Most distinctive of all quartz glass characteristics are their thermal and UV transmission abilities. Unlike other glasses that deform and/or melt at temperatures in excess of 1200°C, quartz glass maintains a rigid shape. In addition, quartz glass has an extremely low thermal coefficient of expansion (approximately 5.0 x 10^-7 Delta cm/cm/°C), meaning that it can withstand thermal shock that would likely shatter all other glasses. Furthermore, although all transparent materials limit various frequencies of light, quartz glass has the potential to transmit the broadest spectrum of light frequencies. However, not all types of quartz glass transmit light equally well: Basically, the purer the material, the better the UV transmission.
As just stated in the above paragraph, not all quartz glasses are alike. Although quartz glass is often called pure SiO2, it can also contain impurities such as alkali metals, hydroxyls, and oxides. These impurities come from the raw materials and/ or manufacturing process. Although these impurities typically are less than 1% and can extend down to the ppb (parts per billion) range, they affect the characteristics of the quartz glass.
The manufacturing processes that limit and/or eliminate these impurities are costly. Thus you select quartz glass first on a basis of intended use^ then on the basis of cost. For example, fused silica with a high hydroxyl (OH ) content will have a significant transmission drop at 2.73 urn in the infrared range. On the other hand, if the infrared range is being absorbed, less energy is required to fabricate items from this type of quartz glass, resulting in lower manufacturing costs.
Currently, either extremely pure quartz crystals (sand) or silicon tetrachloride (SiCl4) are the raw materials from which quartz glass is made commercially. Sand must be separated, sometimes by hand, to exclude any particles with obvious impurities. Then, through one of four heating techniques, raw SiO2 is melted and formed directly into tubes, rods, or crucibles, or it is formed into large solid ingots of quartz glass for later manufacturing.
There are four types of manufacturing processes for quartz glass (Table 1 shows a categorization of commercially available quartz glasses):
1. Type I: Natural quartz is electrically heated in a vacuum or inert atmosphere (at low pressure). This glass is low in hydroxyl content but high in metal impurities.
2. Type II: Natural quartz is heated in a flame. This glass has about the same metal impurity levels as Type I but a much higher hydroxyl con tent.
3. Type III: Synthetic quartz is heated in a flame (for example, an oxy hydrogen flame). This glass is extremely high in hydroxyl content but very low in metal impurities (except Cl, which can be as high as 50 ppm).
4. Type IV: Synthetic quartz is electrically heated. This glass has an extremely low hydroxyl content, but with the absence of hydroxyl, chlorine is increased.
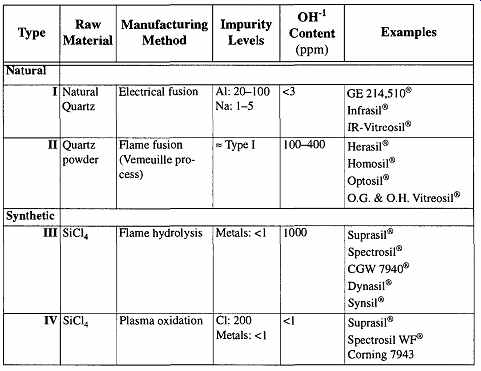
Table 1 Categorization of Commercially Available Vitreous Silicas
There is one unique type of high-temperature glass, manufactured by Corning, called Vycor. Rather than starting out with (essentially) pure silicon as the core material, Vycor starts out as a borosilicate glass with a slightly greater amount of boric oxide than 7740 glass. Then, using phase separation by extended annealing at very specific temperatures , the glass is separated into two glassy phases. Once the phase separation is complete, the glass is particularly vulnerable to attack by water and acids, this allows the leaching out of the soda and boric oxide, leaving about 96% pure SiO2. Then, by heating the porous remains to 1200°C, the material shrinks to a nonporous glass with a coefficient of expansion of 7 x 10^-7 delta cm/cm/°C, making it essentially as resistant to thermal breakage as quartz glass.
Vycor will deform and melt about 100°C lower than fused silica, and it is a poor transmitter of uv light. In the early years of Vycor production, Vycor was significantly less expensive than pure quartz glass. However, as the manufacturing techniques of pure quartz glass have become more efficient, Vycor is now the more expensive material.
It would seem that there would not be much of a market for Vycor because fused silica has (seemingly) better properties and is less expensive. However, "better" has always been a relative term, and this case is a classic one of such relativity. It turns out that some of the undesirable properties of Vycor actually become assets. The lower temperature required to soften and/or melt Vycor means that less energy is required to form and shape the material. Also, Vycor maintains its liquid state over a wider temperature range than fused silica,* thus making it easier to fuse it to other Vycor or fused silica items.
[In a narrow temperature range, fused silica becomes soft, melts, and then volatilizes. Because it maintains a relatively high viscosity once melted, it does not fuse easily.]
Vycor has a thermal coefficient of expansion of 7.5 x 10^-7 delta cm/cm/°C and can be fused directly to fused silica. This property has provided an excellent technique for fusing a borosilicate glass to a quartz glass. Normally, to make such a seal, a combination of three intermediate glasses fused between the outer two is required.
The union of each intermediate glass is under strain, albeit within a tolerable range. However, during Vycor manufacturing it is possible to remove the non SiO2 materials using a controlled, tapered process. This creates a section of glass which can provide an infinitely graded seal between fused silica and borosilicate glass with no significant strain.
In addition, Vycor can be shaped and/or formed while in its borosilicate state before it is transformed into Vycor. Thus, molded, pressed, tapered, and other shapes that would otherwise be very difficult, expensive, and/or impossible in fused silica can be done (relatively) easily with the pre-Vycor material with much less energy. Once the manufacturing is complete, the glass can then be processed to Vycor.
Because Vycor already carries about 4% impurities, it is safe to "dope" Vycor to obtain characteristics such as color or UV opacity. Any similar doping of fused silica would alter the characteristics that pure silica strives to achieve.
Finally, Vycor devitrifies far less than fused silica. Therefore, if you do not require ultrapure baking environments (similar to those demanded in the silicon industry), furnace tubes made from Vycor may be cheaper in the long run than those made from less expensive fused silica.
1.6 Grading Glass and Graded Seals
Grading glass, made from a wide variety of materials, is used to seal glasses with different thermal coefficients of expansion (see Table 2). For example, you can not fuse a pipette directly onto a round-bottom flask because the thermal coefficient of expansion for the pipette is about 51 x 10^-7 Delta cm/cm/°C and the thermal coefficient of expansion for the round-bottom flask is about 32 x 10^-7 delta cm/cm/°C.
This range of expansion is too great for a direct seal, and the stress created at the seal will cause failure at the union. However, if a third glass, with a thermal coefficient of expansion of about 40 x 10^-7 delta cm/cm/°C, is introduced between the two, the stress between the round-bottom flask and the third (grading) glass is within tolerable limits, as is the stress between the third (grading) glass and the pipette.
The strain is split into smaller "steps," which will permit the final product.
Graded seals can be used to join not only different borosilicate glasses, but different types of glasses (and even metals) of different thermal coefficients of expansion. Obviously, it is most efficient and desirable to select glasses and metals with close thermal coefficients of expansion, because it reduces the amount of stress with which the glassblower must deal.
Metals can be sealed directly onto glasses even though they may have radically different thermal coefficients of expansion. For example, copper or stainless steel can be sealed directly to standard laboratory borosilicate glass. However, to do this sealing, the metal must be machined so thin that any expansion is so (relatively) small that it doesn't overwhelm the glass. Unfortunately, by making the metal so thin, it becomes mechanically weak. Another option is to use Kovar® (an alloy composed of cobalt, iron, and nickel), which has a relatively low thermal coefficient of expansion. Although it requires two grading glasses to seal it to common laboratory borosilicate glass, Kovar does not have to be machined thin and therefore maintains greater strength than do machined metals. Often the advantages far outweigh the extra effort involved in making the graded seal.
Kovar is easily soldered or welded to other metals for vacuum-tight seals.