AMAZON multi-meters discounts AMAZON oscilloscope discounts
WE know you expect us to tell you all about transistors (and we will) but let us start by taking a slight detour and talking about nails. What is a nail? Just a bit of iron, shaped in a special way and intended for a special purpose.
But when we say a nail is iron, we're indulging in what is known as a half-truth. Iron is a very sociable sort of metal and very rarely will you find it all by itself. For the most part, it likes to associate with oxygen, and when it does, we call the partnership iron oxide (rust). This union of iron and oxygen is a very strange one. By itself, oxygen (at our usual temperatures) is a gas. We take in a lungful every time we inhale. And it is highly unlikely that you have ever seen pure iron, or that you would recognize it as iron, if you did.
Pure iron is as white as silver, malleable and ductile. All this means is that it is so soft you wouldn't dream of using it to help build a house. But join iron and oxygen, add a pinch of carbon, and what a difference you get. You now have a strong, dark metal with which you can build houses or bridges.
Elements What man hath joined together, man can put asunder. We can take iron oxide, separate the two partners, and have our pure iron (soft and silvery) and our oxygen (a gas) once again. Iron and oxygen belong to a family of substances called elements. Elements form a very exclusive society, since there are only about a hundred of them, but their importance is far out of proportion to their numbers, because they form the building blocks of the universe. Everything you wear, eat or drink, everything you see, including yourself and the people around you, is made of elements.
Some you know very well or, at least, their names are familiar. You know arsenic and aluminum, calcium, chlorine and carbon, helium, hydrogen and iron, but there are some that, until recently, were not so publicity-conscious. But we are going to stir up these very shy ones-germanium, selenium and silicon, indium, boron and gallium-and give them the sense of importance they deserve.
Compounds and mixtures
Fig. 101. Alcohol is just one of the many thousands of compounds that
can be formed from carbon, hydrogen and oxygen.
Elements, like people, have very distinctive characteristics. And, like people, they may change these characteristics when they get into a group or they may remain as individualistic as they ever were. Iron and oxygen unite to form iron oxide, a substance that is as different in its characteristics from iron and oxygen as it could possibly be. Sodium, a soft, silvery-white, waxlike metal, joins enthusiastically with chlorine (a nasty gas) to form sodium chloride. We know it much better as ordinary salt and we need it as part of our diet. Carbon, hydrogen and oxygen, having rid them selves of their inhibitions, group together to form alcohol (Fig. 101). Add some coloring matter, invite a few congenial people,
and we have ourselves a party. But how different carbon (think of coal or diamonds), hydrogen and oxygen (both gases) are from alcohol.
This grouping, or combination, of elements to form something completely new, something with entirely different characteristics, is called a compound. Water is a compound, made of hydrogen and oxygen. Salt is a compound. And so is iron oxide.
Does this mean that the penalty for being sociable is loss of identity? Not always. We can take one compound, such as salt, shake it together thoroughly with another compound, such as pepper, but neither compound, pepper or salt, will change its characteristics. The pepper remains sharp or tangy and the salt remains salty. A loose union of this sort in which the substances retain their individualities is called a mixture.
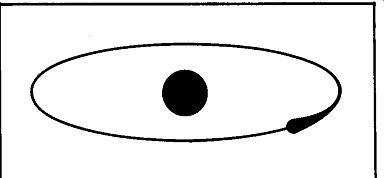
Fig. 102. The lonely world of the hydrogen atom. The nucleus has just
a single orbital electron to keep it company.
Atoms
Now that we've moved somewhat in one direction, the joining of elements, let's make an about face and start marching off in the other. What happens when we start slicing elements apart, finer and finer and finer. . . ? The end product is an extremely tiny particle of the element, known as an atom. We say end product because, if we tamper with the atom in any way, it loses its
identity. An atom of pure iron behaves in the same way as any other atom of pure iron, but start taking one of them apart and it is no longer an atom, and it is no longer iron.
Actually, though, the atom is as far as we are going to go.
Taking the atom apart is the role of the atomic physicist. We are going to dally in that very pleasant and instructive playground barely long enough to get some basic information about transistors.
Just about the simplest of all the atoms we could start with is the atom of the element hydrogen. Fig. 102 is a drawing of what we think an atom might look like. Note that this atom has only two parts-a central body called the nucleus and a single, solitary electron revolving around it. This electron, in its motion around the nucleus, follows a definite orbit.
An atom is matter-mighty small, but it is matter, and as such it has mass. This applies both to the central part of the atom, the nucleus, and to its restless associate, the electron. Oddly enough, although the nucleus of the hydrogen atom has a mass that is about 1,845 times that of the electron,' it is the electron that we are interested in. It is the diminutive member of this duo, the electron, that lets us have radio and television, that is responsible for modern communications.
======
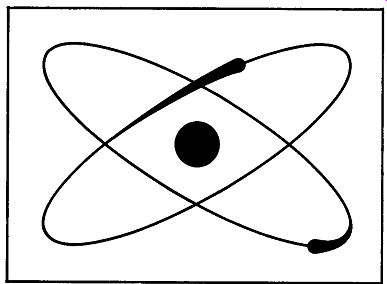
Fig. 103. The helium atom is content with its two orbital electrons.
The helium atom is stable.
[1. The electron has a mass of 9.107 X 10-'" gram. The diameter of an electron is believed to be 2 X 10^-12 centimeter. All electrons are identical, whether free or part of an atom.
2. Each electron has a charge of 4.80 X 10^-1 C.G.S. electrostatic units.]
======
There are a few things we know about the electron and there are quite a few things about the electron that have us guessing.
We know it has a negative charge, and has a magnetic field around it. It is possible that the electron has angular momentum-that is, it spins or rotates around an axis. This knowledge of the vital statistics of the electron is singularly useful because it enables us to persuade the electron to leave its nice, comfortable orbit and to go traveling for our benefit. Specifically, we have transistors be cause we are able to make electrons do our bidding.
In the matter of electrons, hydrogen is the poorest element, since it has but one. The next element, helium, has two orbital electrons (Fig. 103). But now comes a sad story-a story of envy, desire and greed, the story of the haves and the have-nots. An atom with two orbital electrons (and helium is an example) is a satisfied, contented atom. It has no ambition. It has no desire to acquire more electrons and, even if offered some, will reject them.
But what about hydrogen, poverty-stricken hydrogen, with its single electron? It schemes day and night to form a stable, happy family group, so reminiscent of helium. But, electrons are not easily come by, and so the hydrogen atom shrewdly does the next best thing-it joins forces with other hydrogen atoms, just as shown in Fig. 104. Each hydrogen atom shares its electron with its neighbor, and so, in that sense, we can say that the hydrogen atom has finally satisfied its yearning to have two electrons. But what about. atoms (see Fig. 105) that have more than just one or two electrons? Fig. 104. Two hydrogen atoms can satisfy their yearning for stability by sharing electrons.
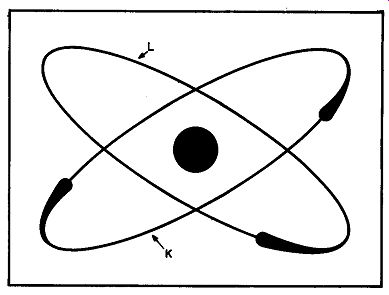
Fig. 105. The lithium atom has three electrons in two or bits. The two
innermost electrons form an orbit or ring of their own. This is known
as the K ring. The remaining electron has a separate orbit called the
L ring. The single electron is much further removed from the large, centrally
located nucleus, than the two electrons in the K ring.
We don't know why two electrons revolving around a nucleus makes the atom satisfied, but it does. One hint lies in the fact that the next atom, lithium, has three electrons. Two of lithium's electrons play in an orbit of their own which we have marked with the letter K in Fig. 105. But what about the third electron? It must revolve in an orbit all by itself. We have marked this orbit with the letter L. Is there anything about the lithium atom that seems familiar? Doesn't it seem to you that there is some resemblance to our smug, self-satisfied helium atom (two electrons), and also to the dissatisfied hydrogen atom (with just one electron)? Actually, such is the case. The electrons in the K group are complete but, give it half a chance, and the lithium atom will associate with some other atom just so that its outermost electron (the single electron in the L ring) will have a playmate.
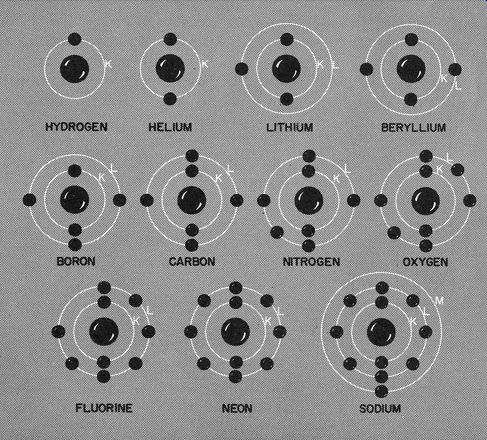
Fig. 106. One of the differences between atoms is the addition of electrons
in the various rings. Hydrogen has one electron, helium has two, lithium
three, etc.
Other Elements
There are far too many elements for us to describe each one in detail, but Fig. 106 gives some indication of what is happening.
As you can see, one big difference between atoms is that each succeeding element has one more electron.
There is something even more important that Fig. 106 is trying to tell us. The electrons seem to want to arrange themselves into definite orbits. We call them shells, because they surround the nucleus or kernel. The first; or K, shell has 2 electrons. The next, or L, shell is filled when it has 8 electrons. The next shell (M) calls for 18 electrons and when we move along to the N shell it wants 32 electrons.
But what if an atom does not have 2, 8, 18 or 32 electrons? What does it do? It follows the pattern set up by the hydrogen atom and shares electrons, or, as we shall see, it will surrender electrons, provided we can supply the atom with some sort of guarantee that it will get other electrons in return.
Please pass the electron
As you have probably noticed by now, we're spending quite a bit of time talking about electrons. This is exactly as it should be, since electrons are our stock in trade. We're not really interested in all electrons, just those we can persuade to leave home and travel. Like people, some electrons can be influenced to do what we want; others cannot. And since this is so, let's separate those who will from those who won't.
If a shell, or ring, of electrons is complete-that is, if the ring contains 2, 8, 18 or 32 electrons, don't even bother trying to talk them into leaving. These are the home bodies. We have a few of them in Fig. 106. Helium is one of this group. It has one ring (K) of 2 electrons, is a stand-patter, and that's that. The next one is neon with a total of 10 electrons. No chance of borrowing any electrons here, since the K ring is complete with 2 electrons and the L ring is filled with its quota of 8.
Some electrons move--some electrons won't
Now you can begin to appreciate why we started our study of semiconductors with atoms and why we did not plunge immediately into an explanation of transistors. We're interested in get ting electrons to move when we tell them to. A small knowledge of atomic structure is helpful because we know in advance what elements will be cooperative and which elements will be stubborn.
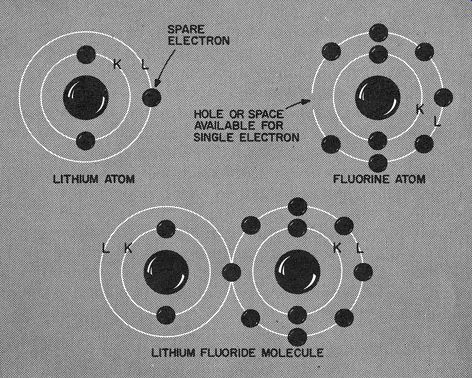
Fig. 107. Lithium has an electron it can spare. Fluorine has a vacant
space into which this electron can fit. The two atoms, lithium and fluorine,
form a molecule of lithium fluoride. This is a chemical combination.
The molecule of lithium fluoride is a compound that has its own characteristics,
completely different from that of either lithium or fluorine.
Fortunately for us, very few atoms are as complete as helium or neon. Most of them have electrons that jump about almost as fast as women at a bargain hunt at a charity bazaar. Let's examine two of them, shown in Fig. 107. Here we have lithium and fluorine.
Lithium's inner ring is 2 electrons, so we don't bother with it. But that outer electron has an itch to travel. Adjacent to the lithium atom, we have one of fluorine. Fluorine also has an inner ring of 2 electrons, but its outer ring has a juicy total of 7.
How do lithium and fluorine compare? Lithium is "electron poor." It has just I electron in its outer ring and not much opportunity to reach a grand total of 8. But what about fluorine? Its outer ring has almost made its quota. Just one more little electron and the L ring of fluorine is complete.
Now if we bring lithium and fluorine near each other, just what do you think will happen? Fig. 107 gives us the result. The single, solitary, out-in-space electron of lithium moves over to fluorine.
This union of lithium and fluorine gives us a molecule" which we call lithium fluoride.
We're not interested in teaching you chemistry-that is quite incidental to this book. The important thing to learn from Fig. 107 is that we now have one way of making an electron move. We don't say it is the best way (for us), but it does show us that electrons do get about.
Of even greater importance, Fig. 107 illustrates which electron moves. It is the electron in the outermost ring or orbit. And be cause it is so important, so fundamental to our work in transistors, let us examine this union of atoms with another example.
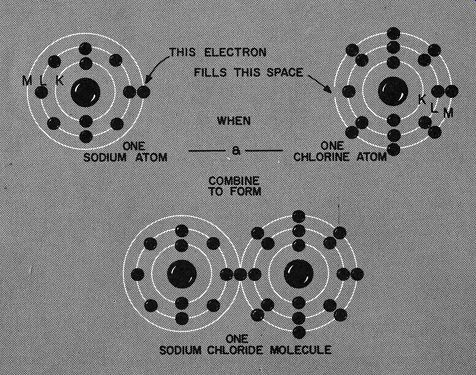
Fig. 108. The sodium atom has an electron it can spare in its outermost
ring (M) while the chlorine atom has room for one more electron in its
outermost ring (M). The union of these two atoms produces a molecule
of sodium chloride.
Fig. 108 is a drawing of a sodium atom. This atom has three rings, two of which we cannot touch. The first, or K ring has the usual 2 electrons, the next or L has a complete and very satisfying full quota of 8. But orbiting out in the space around the nucleus we have a single electron in the M shell.
• A molecule is the union of 2 or more atoms, forming a compound.
In the same drawing we have pictured the chlorine atom. This also has three rings, two of which are filled. The M ring, though, has 7 electrons. And, as in the case of lithium and fluorine, the one electron in the outermost shell moves over. The result of this very fortuitous joining of sodium and chlorine is sodium chloride.
Other combinations
We selected lithium and fluorine, sodium and chlorine, because they lent themselves so nicely to the idea of a moving electron. But other atoms join in just the same way. In each case, though, the only electrons we are interested in are those that can (and do) move, and these are always the electrons in the outermost orbit.
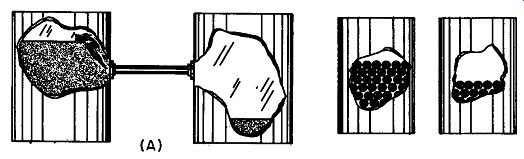
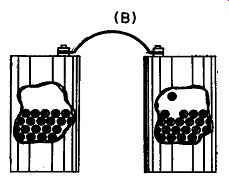
Fig. 109. In drawing A we have two containers, one of which has a higher
water level than the other. If we connect a pipe between them, water
will flow until both containers have the same quantity of water. In drawing
B we have two containers, one of which has more electrons than the other.
If we connect them with a wire, electrons will flow until both containers
have the same number of electrons.
This is true whether the atom has two shells or more than two.
And because of this, we can simplify matters for ourselves in talking about other atoms by forgetting all electrons except the outer most ones.
Electrons in motion
Getting electrons to move is easy. To get the picture, we can compare their motion to the flow of water, but we must bear in mind that this is not a really correct description. In Fig. 109-A we have two cans of water, one of which has a higher water level than the other. Connect the two with a pipe and water will flow until both cans have the same water level. Now let's try idea with electrons. In Fig. 109-B we have a container and let us just imagine that we have somehow managed to fill it with electrons. Standing nearby is another container with far fewer electrons. And connecting the two is not a pipe, but a copper wire.
Although we have no way of seeing directly what is going on, electrons will move from the electron-rich container to the one having not so many, until both have the same amount.
Now you might argue that it isn't possible to have two containers, one with, and the other without, electrons. But any battery presents almost that situation. A battery is a chemical factory, the end product of which is the condition whereby one electrode is loaded with electrons while the other has a terrible shortage of them. And if you wonder how it is possible for such a situation to come about, just remember that a battery is a very convenient arrangement for robbing some atoms of their outermost electrons.
Some atoms are constantly losing electrons, some are always gaining them, and some (as in the case of neon or argon) never swap.
Conductors The copper wire we connected between our two containers of electrons, or the terminals of our battery, is called a conductor be cause it supplied a passageway for electrons. Copper is another one of those elements whose atoms have very unsatisfied outer rings. Copper has four rings, 2, 8, 18 and 1 electron. This last ring, or shell, is so empty that it accepts all contributions with gratitude.
Now suppose we connect our copper wire to the negative terminal of a battery. Can't you just see the very first atom of copper saying to the electrons, "Come over and join my outermost ring"? And so they do. But the copper atom adjacent to the first one is also unsatisfied, so it demands, and gets, the electrons. This leaves the first atom shy of electrons again, but why worry? There are more available. And so the electrons are handed along, from atom to atom, much like buckets of water passed down the line by a 17th century fire brigade.
Now let's move over to the very last atom of copper, the one nearest the positive terminal of the battery. Do you think the last atom on the line is going to be allowed to hold on to its precious hoard of newly acquired electrons? Hardly! The positive terminal of the battery has even fewer electrons than this atom, and so it demands, and gets, its share.
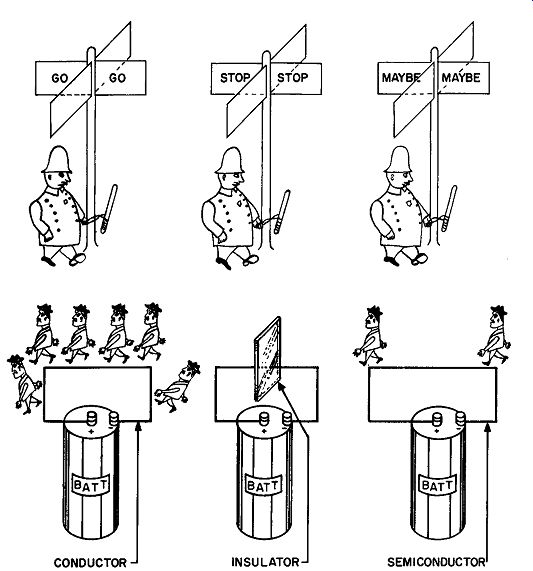
Fig. 110. A conductor permits the passage of electrons; an insulator
does not.
A semiconductor comes somewhere between these two extremes.
When will this sort of thing cease? When the positive terminal of the battery has as many electrons as the negative terminal. Of course, at that time, there will no longer be such a thing as positive or negative terminals, since both will have the same number of electrons. And what about our copper wire? Will it have been permitted to keep any of the electrons it was so busy passing along? Sorry. The battery gives electrons and the battery takes them away.
Our copper wire, willing and gullible, ready to work without electron compensation for any slick battery that comes along, is called a conductor. A conductor permits the easy passage of electrons through it (Fig. 110).
Insulators
Once a compound has acquired all the electrons it will ever need, any attempt we make to supply it with some will be re buffed. Generally these are compounds whose outer rings have joined and so wouldn't know what to do with extra electrons if offered on a silver platter. Glass is one such compound. It has all the electrons it needs, thank you, so connecting a battery across it is a nice gesture, but a useless one.
Semiconductors
Substances that are very good conductors or very good insulators are unique and represent a minority group. They belong to the all-or-nothing-at-all groups. We can take certain insulators, modify them somewhat to form a let's-straddle-the-fence-group, which we call semiconductors. It is from this group that we are going to build our transistors.
The trouble with oversimplification (which is what we tried to do in the paragraph above) is that it can lead you down the garden path to some incorrect thinking. An ideal insulator could be rep resented by an open circuit, or an infinite amount of resistance.
An ideal conductor could be considered as a short circuit, or zero resistance. Between these two limits we have a tremendous number of resistors which are able to pass current to a greater or lesser degree. We can call them resistors or we can call them conductors, just as we prefer. They do not, however, include semiconductors.
A semiconductor is not something that is halfway between an open circuit and a short circuit. As we mentioned in the preceding paragraph, a semiconductor starts out in life as an insulator and is then modified to give it certain characteristics.
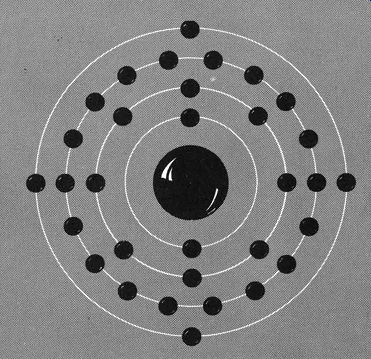
Fig 111. The germanium atom has 4 electrons in its outermost ring.
Back to the electron
Germanium, a grayish-white, brittle sort of metal is among the more important semiconductors. In some ways it is like carbon or silicon and, in others, like tin. Germanium is a mineral and, like almost all other minerals, has a crystalline structure.
Salt is also crystalline. Look at it under a magnifying glass some time and you will see how each face of this mineral has a definite geometric pattern. This doesn't mean that salt and germanium look alike, any more than two hats. Each mineral has its own definite structure.
Germanium has four electron shells, 2, 8, 18 and 4. It is the outer ring that fascinates us, since it is responsible for some rather unusual behavior. Actually, having four electrons in the outer most ring is what helps make germanium unique. It really doesn't fit in with those other atoms, like lithium, which loses electrons readily, or like fluorine, which obtains electrons so easily. Germanium is a sort of halfway point between atoms that surrender electrons and those that take them in. We have a drawing of a germanium atom in Fig. 111 and as you can see, the atom is sort of at loose ends with its incomplete outer shell.
But what will happen if we introduce our germanium atom, not to some stranger, but to some other germanium atom, also beset with the same problem. Which of the two will surrender its electrons? Which of the two will be the more demanding, and get electrons? What we have is a tug-of-war between equally matched contestants. Neither side wins and neither side loses.
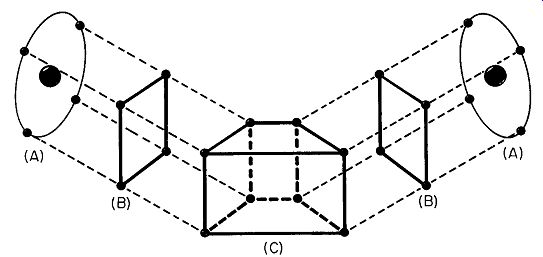
Fig. 112. In A we have a pair of germanium atoms. Just the outermost
ring of electrons is shown. We can imagine these removed from the atoms,
as in B. In C we have the 8 valence electrons, 4 from each germanium
atom, forming a bond. This is atomic union, not chemical, as in the case
of sodium and chlorine.
Valence
Up to now we have really been talking about two kinds of electron behavior. We have the stay-at-home, stick-in-the-mud types that form the ultraconservative complete rings or shells. And we have the let's-go-traveling-and-see-what-other-atoms-are-up-to types. But even here we must make some sort of distinction, since not all of the electrons in the outer incomplete rings are truly and equally venturesome. Let's go back to the case of lithium and fluorine. Lithium has 1 electron in its outermost orbit and fluorine has 7. Should they all get equal credit for having some git up and go? Not likely! There is just one electron that does any moving, so we should distinguish it in some way. We can, by giving it a new name. We can call it a valence electron.
But having endowed the lithium electron with this verbal croix de guerre, what shall we do about germanium? Fig. 112 shows what happens. The electrons don't move from one germanium atom to the next, but they do sort of link hands. Technically speaking, each germanium atom now has a complete outer ring of 8 electrons. True, it gets this way by a sharing process, but the ring is still complete and that is just what the atom wants. While the electrons didn't move, they do deserve some sort of special recognition, so let us say that they have formed a valence bond.
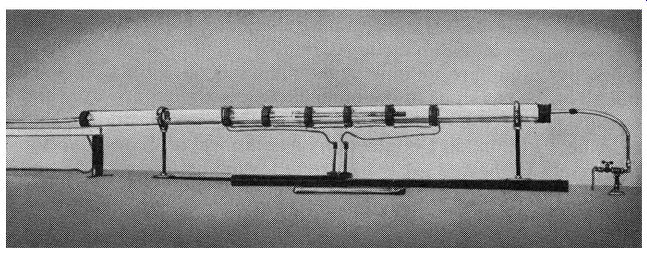
Fig. 113. Technique for refining germanium. This uses a method known
as zone refining. The coils produce heated zones in succession, the impurities
being pulled to one end of the tube. If the refining is repeated often
enough, the amount of impurities remaining will be less than I part in
I billion. (Bell Telephone Laboratories, Inc.)
Back to conductors
A little earlier we connected a copper wire across a battery and saw how we could get an electron movement, or drift, through the wire. We were aided by the fact that the outermost rings of all the copper atoms were incomplete and were happy to get electrons from the battery, even if only on a highly temporary loan basis.
Now let us repeat our experiment, but this time let us make our wire out of germanium. We connect our wire to the battery and stand back expectantly, but nothing happens. Why? Our battery is fresh, its negative terminal is practically crawling with electrons and its positive terminal is suffering from an electron famine.
Conditions are just ripe for the passage of electrons through the germanium wire-or are they? Why should the germanium wire pass electrons? What have the germanium atoms to gain? Absolutely nothing. Linked arm in arm by mutually shared electrons--by valence bonds--they are self-sufficient, and have no need of electrons. No current will flow and we have no choice but to classify germanium among the insulators. A rather odd situation, since germanium is a metal.
Down with the valence bonds
Possibly you find the idea of germanium as an insulator slightly irritating. But if so, what can be done about it? One method (and we do not offer it as a practical technique) is to heat the germanium. As the temperature rises, some of the valence bonds weaken.
Some of the outer rings assume an attitude of haughty aloofness. In this condition they have only 4 electrons and will welcome electrons from the battery. Later we will learn that this tidbit of knowledge is useful in protecting the transistor and keeping it, lemming-like, from attempted suicide.
Fig. 114. The atom of antimony has 5 electrons in its outermost ring,
as in A. The inner rings have been omitted to simplify the drawing. We
can connect the electrons as shown in B. Antimony has I electron more
in its outer ring than germanium.
Add a pinch of this or that and stir thoroughly The germanium we have been talking about so far is absolutely pure. You might argue that an absolutely pure element does not exist, but in the case of germanium at least, methods are known and used which make germanium 99.99999 (add a few more nines of your own) percent pure (Fig. 113). But now, into this undefiled mass of germanium atoms, let us drop a tiny impurity. Did you ever see a kitchen floor that was absolutely spic and span, but with one tiny, muddy footstep? What did you notice-the whole expanse of clean floor, or the small spot of mud? We're not going to use mud for our germanium but we can add a different element to it, and because this element is different, we can call it an impurity. (The process of adding the impurity is known as doping.) In Fig. 114 we have a drawing of the element we are going to use. This element, antimony, has no less than 5 rings, or shells, of electrons. The innermost has the usual 2 electrons, the next has 8, followed by two complete rings of 18 electrons each. And in the outermost shell we have 5 electrons.
As far as the outer shells are concerned, how does antimony compare with germanium? Germanium has 4. Antimony has 5.
Do you remember our description of germanium. Antimony sounds almost like a twin, since it is tin-white in color, and is a hard, brittle metal having a crystalline structure.
Lattice structure
Before we stir up a witches' broth by adding antimony to germanium, let's go back for just a moment to germanium itself and consider an important fact about the construction of that element. Did you ever see a beehive? A marvel of geometric construction, isn't it? And if you could see the way germanium is put together, you would marvel at it too. Germanium has a lattice structure. Whenever we have a definite arrangement of atoms in a geometric pattern, we have a lattice. This is why germanium is crystalline. The most beautiful example of crystalline structure is the diamond. The surfaces of a diamond are arranged sym metrically, but the surfaces are just an indication of the extremely orderly arrangement of the atoms inside.
Atoms, like people, can come in clumps or bunches without any sort of order or plan. Think of people walking back and forth, and cross-wise along a pavement. Or, people can form orderly groups, as in a well organized parade.
Adding the antimony
Now antimony is a crystalline substance too and so, when we put a tiny speck of it in with the germanium, we are adding one element with a lattice structure to another with a similar lattice structure. All well and good, but there's an electron in the ointment (Fig. 115). If antimony had only four outermost electrons instead of five, all would be fine. Four electrons-yes, but that fifth electron is about as necessary as an extra guest when all the chairs are filled. But that extra electron, that orphan, should not be over looked, for now it can serve a useful function. Now you might think that the antimony would want to keep its number 5 electron, but such is not the case. Its lattice structure fits right in with the germanium. It can form valence bonds with four of its outer electrons. What is the antimony doing? It has gone native, hasn't it? It no longer needs or wants its surplus electron and is quite willing to release it and let it go elsewhere.
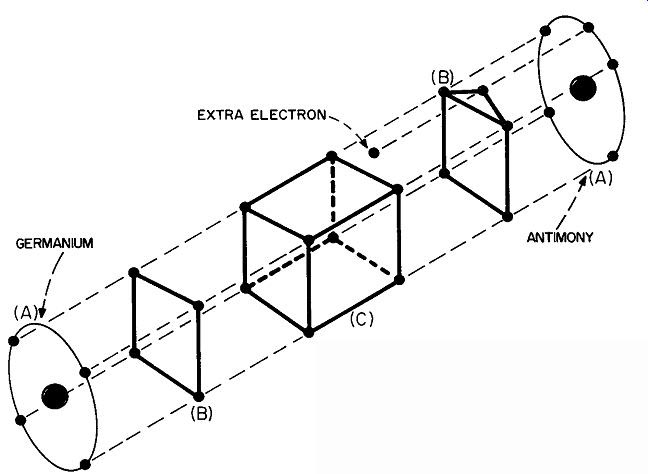
Fig. 115. When antimony is added to germanium, the result is a lattice
structure-a bond between the germanium and antimony valence electrons.
There is one electron, though, that isn't strongly bound.
Now we can try our experiment once again, but this time let us use a wire made of germanium to which we have added a tiny amount of an impurity-antimony. We connect this wire across our battery. This time the electrons on the negative terminal push over onto the wire. The extra electrons of the antimony atoms, unwanted, are repelled by the electrons from the battery, and edge their way over to the positive terminal. But as soon as an electron leaves the outer ring another one hops in to take its place, only to be displaced by another electron moving forward toward it. But where have we heard this story before? Isn't this somewhat like the movement of electrons along the copper wire? Our germanium is now no longer in the insulator class. At the same time, we really can't claim that it belongs with the good conductor group of which copper is such a prominent member.
Since it is in a sort of limbo between the two, we call germanium, doped with an impurity such as antimony, a semiconductor.
Other impurities
The person who tracked up our nice clean kitchen floor a few paragraphs ago could also have brought in other impurities.
Similarly, we need not depend on antimony alone. We could also use phosphorus or arsenic. Phosphorus has only three electron rings, but the outermost one has 5 electrons. Arsenic has four electron rings, but once again the outermost shell has 5 electrons.
Unfortunately, we don't have an unlimited supply of elements with 5 electrons in the outer shell. Bismuth is the only other one. It has no less than six rings, but it still meets our requirements.
The donors
These elements we have been talking about, antimony, arsenic, phosphorus and bismuth, always come bearing gifts. The gifts are the extra electrons so essential if we are to make germanium into a partial conductor. We call these elements donors, since they really do come equipped with a present of an electron. And since electrons are negative, we really should call them negative donors. Of course, the germanium, recipient of extra electrons, should be called negative germanium to distinguish it from germanium that has retained its essential purity. And negative germanium it is, abbreviated as n-type germanium.
The union of germanium and a donor element such as antimony or arsenic isn't at all like the joining of lithium and fluorine we talked about earlier. Lithium and fluorine combine chemically to form a completely new compound. An example with which you are more familiar is the formation of salt (sodium chloride) from sodium and chlorine. The salt doesn't look at all like its for bears, doesn't act like them, and is the true chemical union called a compound. But let's get back to germanium and the antimony.
We add some antimony to the germanium but no new compound is formed. The germanium remains what it is and so does the antimony. The intermingling of the two is on an atomic basis. The nearest analogous situation we have in practice is putting butter on hot pancakes. The butter melts and mixes with the pancake until every tiny available space is filled with it. But the pancake remains a pancake and the butter is still butter.
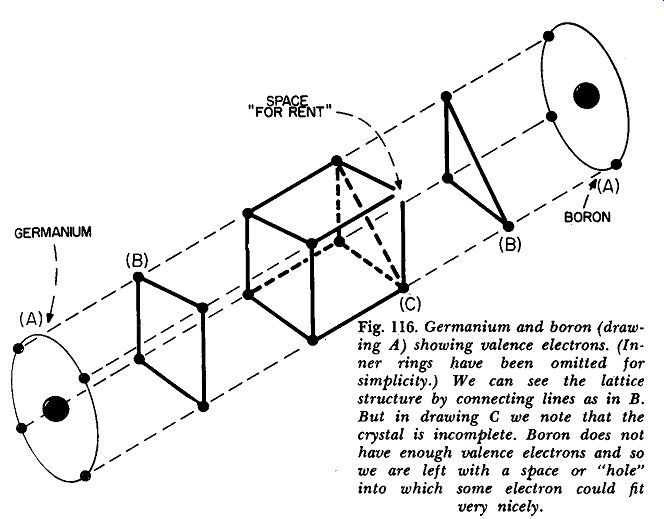
Fig. 116. Germanium and boron (drawing A) showing valence electrons.
(Inner rings have been omitted for simplicity.) We can see the lattice
structure by connecting lines as in B. But in drawing C we note that
the crystal is incomplete. Baron does not have enough valence electrons
and so we are left with a space or "hale" into which same electron
could fit very nicely.
The acceptors
Did you ever see the number 4 written as 4 ± 1? What this means is that we can have 4 plus 1 (to give us 5) or 4 minus 1 (resulting in 3). What has this to do with us? We've been talking about germanium atoms with 4 outer shell electrons and about donor elements all of which have 5 outer shell electrons. In other words, 4 plus 1. This gives us a clue to the idea that we might intermingle another type of element with our germanium. This element would have only 3 electrons in its outer shell.
What elements could these be? Boron is one of them. Boron has just two shells, a complete K shell having the usual 2 electrons, and an outside shell of 3 electrons. Aluminum is another one but has three rings; 2 electrons for the first, 8 electrons for the second and 3 outermost electrons. We have to travel down a long list of elements to come to the next one-gallium, with four electron rings: 2, 8, 18 and 3. The next is indium with five rings: 2, 8, 18, 18 and 3. Then we have thallium with six rings-2, 8, 18, 32, 18 and 3.
And that is all. There are no more elements with 3 electrons in the outside shell, but we have enough now, and any one of those we have mentioned is suitable for our purpose.
Now let us diffuse some boron into pure germanium. Did you notice that we italicized a word? We did so deliberately because the process of getting some boron into the germanium isn't just an ordinary mixing operation. The effect is shown in Fig. 116.
Of course, the boron atom, being part of a minority group, would like to do anything and everything to make people think that it is not a boron, but a germanium atom. But to be able to carry out this deception it needs just one electron, which it promptly borrows from its nearest neighboring germanium atom.
But this germanium atom, having lost an outer electron to the predatory boron atom, promptly borrows an electron from the nearest adjacent germanium atom. And so it goes. Not a single atom wants to be short that single electron in its outer ring, and so we have a sort of aimless wandering of electrons from one atom to the next.
Positive and negative charges
Earlier we mentioned that electrons are not only particles of matter (but extremely tiny) but that they also carry a negative charge. Of course, the more electrons you manage to collect into one spot, the more negative that spot is. Suppose we do have such a location and that four electrons are sitting there, just as nice and comfortable as you please. And now, while we are using our imagination, let us also suppose that one of the electrons gets into a huff and departs. Our location now has fewer electrons.
But precisely because it has fewer electrons, it is not as negative as it was before. And because it is less negative, we can say that it is more positive. It's just like saying that if a person becomes less rich he also becomes more poor. We know you may think this is just a play on words, but it is important for us to realize that we can say one thing in two different ways and to have both of them mean the same.
Holes Let us now consider the case of the unfortunate germanium atom that finds an eager-beaver boron atom located right next to it. Before the germanium atom realizes what has happened, it loses one of its four outer-shell electrons to the boron atom. We know that the germanium is going to steal an electron from its neighbor, in turn, but before it gets a chance to do so, let us "freeze" the picture, so that we can examine the germanium atom as it exists during the time of its loss.
The germanium atom has lost an electron and, since electrons are negative, the germanium atom is now less negative than it was before. But let's define a little more clearly just what we mean by less negative. If your shoe is too tight, it may be just one toe that hurts. You don't ache all over. And when a germanium atom loses an electron, it doesn't become positive all over. We're concerned only with the outer ring, and even there, not with the entire ring.
Consider the region vacated by the electron. It is now positive due to the departure of the electron. The loss of the electron has left a space. We call that space a hole, and to add further to what you must surely think is a state of confusion, some people refer to it as a positive hole.
• This is a redundancy. A hole is positive.
Do you remember our description of a germanium atom? One of its characteristics is that it has 4--not 3--not 2--not 5--electrons in its outermost ring. For a germanium atom to really be a germanium atom, it must have its particular structure, and no other. Remove any part of the germanium atom, such as an electron, and you have made a change, an important change. It is no longer really and truly a germanium atom, since its architecture has now been made different. It is an atom with a positive charge.
But now let us take pity on our electron-deprived germanium atom and supply it with an electron-a negative charge. Where do you think that electron will go? There is only one place it can fit and that is in the "hole" created by the departure of an electron.
If you want, we can say that the hole and the electron recombine.
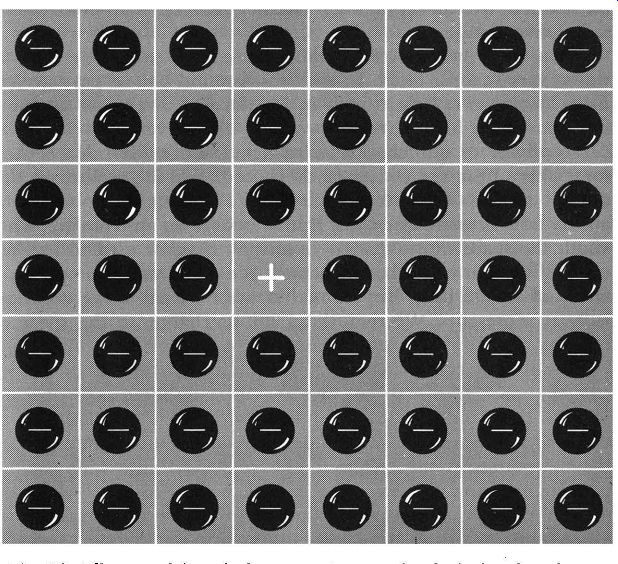
Fig. 117. All we need is a single vacant space on the checkerboard,
and every electron on the board can be made to move. We can visualize
this movement in two ways. We can think of the electrons moving about
on the board, or, equally important, we can think of the space or "hole" taking
different positions on the board.
A strange game of checkers
We want you to imagine that we have a checkerboard in front of us and that we have covered every available space on the board with checkers. This is extremely unusual, but so are transistors, so we'll make up our own rules as we go along. We also want you to imagine that each checker has a big, bold minus sign painted right on it where we can see it. Each checker represents an electron, or a negative charge.
What is our first move? Actually, there is no move we can make since every space is filled. Doesn't this remind you of pure germanium, where every electron is in its place? There's just no room or opportunity for electron movement whatsoever.
Now back to our checkerboard again. Let's lift one checker, somewhere near the center of the board. For the most part this doesn't seem to help very much (Fig. 117) but we do have the ability now to move electrons. But before we do any moving, what about the space where the single solitary checker used to be? We could give it any number of names. We could call it a space, an enclosed area, a hole. But no matter what we call it we do know what it represents. We removed a minus charge, so the space or hole, or whatever we want to call it, is positive." Now let's start moving checkers. If we're patient about it we can make that hole or space move anywhere on the board we want.
We can chase the hole progressively from one end of the board to the other. But as we do a very peculiar circumstance emerges. As the hole moves in one direction, electrons move in exactly the opposite direction. We can call the movement of the hole a movement of positive charge or hole flow. Or, if this represents too great a strain on our imagination, we can still think of electron flow.
We've been away from our germanium for quite a while now, so let's go back to it. Into a bit of pure germanium let's diffuse some boron. There's going to be a bit of an electron scramble as the germanium and boron atoms start their mad interchange of electrons. But, as in our strange checker game, there's always going to be a positive hole somewhere. Actually, we are going to have a tremendous number of holes because just a speck of boron provides millions of atoms. This is going to be a checker game on a really grand scale. We are going to have numerous positive charges, so it's perfectly proper and permissible to call this type of germanium, positive germanium. Let's do some abbreviating and call it p-type germanium.
We now have two types: n-type germanium and p-type. Which we get depends entirely on the element we add to our pure germanium.
The electron not only has a charge, but mass as well. It can exist independently of the atom. A hole, though, is a charge only. It depends for its existence on the existence of the atom. Holes, or positive charges, do not have a life apart from the atom.
Current flow
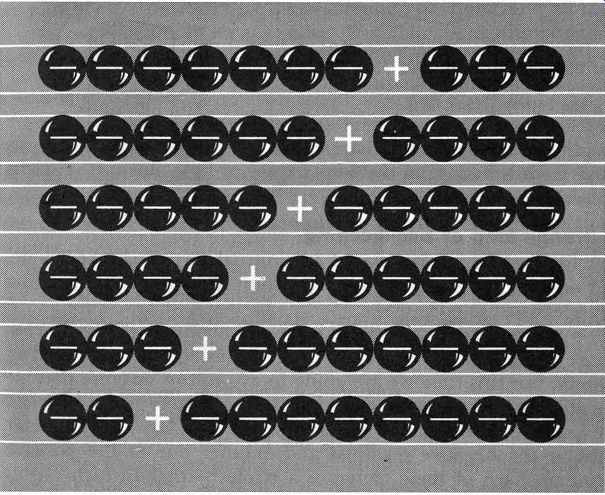
Fig. 118. As the electrons (negative charges) move to the right, the
hole (absence of electron, or positive charge) moves to the left.
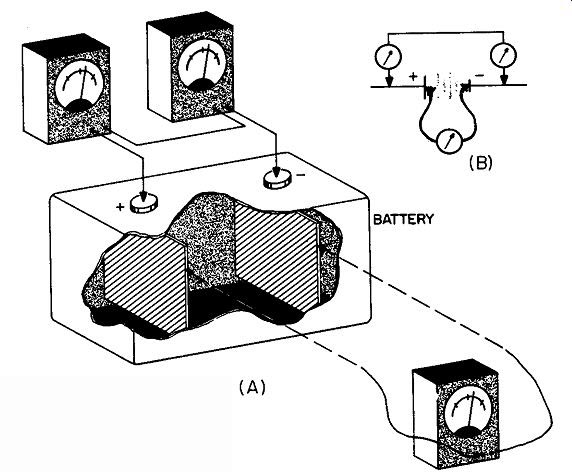
Fig. 119. A perfect bookkeeping system! Electrons are never lost. The
same number of electrons that leave the battery return to it.
When we made a wire of n-type germanium and connected it across a battery, we had a trickle of current flowing, but there was nothing particularly unusual about it. But what about p-type germanium? What will happen when we make a wire of such germanium and connect it across a battery? As usual, the negative terminal of our battery is crowded with untold millions of electrons all of whom are casting longing eyes at the wide open spaces on the positive terminal, so near and yet so far away. When we connect our p-type germanium wire, the electrons on the negative terminal can see but one thing ... holes-- positive charges--just willing and waiting to be filled by electrons. And so the electrons go hopping through the wire, moving along from atom to atom. And while the electrons move through the wire in one direction, we get the effect of the positive holes moving in exactly the opposite direction. If you have any trouble visualizing this, just look at Fig. 118 where we have a simplification of what is happening in our wire. As electrons move from left to right, the empty space or hole moves from right to left.
A strange kind of bookkeeping A little while ago we played an unusual sort of checker game, and now we are going to have an accounting system that is going to appear just as odd.
To understand just what it is we are about to thrust at you, please take a look at Fig. 119. We are still using our long-suffering battery, but this time we are going to ask you to imagine that we are able to insert an ammeter right in the battery itself, between the positive and negative electrodes. We also have an ammeter right at the negative terminal and another one at the positive terminal. Each of these meters is a triplet*- that is, each one is absolutely identical. With our wire connected, each meter will read exactly the same.
[ With apologies to Triplett Electrical Instrument Co.]
Is this so surprising? Hardly! We have a series circuit and so the current must be the same in each and every section of that circuit.
We are sure this isn't front-page news as far as you are concerned, but there is a moral and a lesson to be learned here. For every electron that leaves the negative terminal of the battery an electron must enter the positive terminal. And because of this, our copper wire never changes. When we disconnect it, after electrons have passed through it from the battery, it is precisely the same as it was when we first connected it. The books are balanced. There has been no loss of electrons, nor has there been any gain.
Doesn't this apply to our germanium as well? Obviously a series circuit is a series circuit no matter what components we use, and equally obviously, the battery isn't going to change the way it operates just because we hang some germanium wire across its terminals. Again, what does this really mean to us? Simply this: p- or n-type germanium, like any other conductor, will let electrons enter from a battery, but the total number coming in will equal the same number going out. The germanium is not left with a surplus of electrons. All that the germanium has done, and all that it can possibly do, is to act as a passageway. The germanium doesn't care which element we use as an impurity either.
Current movement There are just a few miscellaneous facts we should discuss before we go into the next section. We've been talking about wire made of germanium simply to convey an idea. It is doubtful if there is such a commodity and, if by some very strange coincidence there should be, it would certainly be lots more expensive than copper.
We've also been talking about the flow of current through cop per and through germanium. The words flow of current may, un fortunately, create a completely wrong impression. The movement of current through a copper wire is not a mad dash. Far from it. It is much more like a leisurely drift, but it certainly does give the impression of being instantaneous.
Consider a circular tube filled with metal balls, just like a ball bearing, from which the idea was taken.
Now let's push one of the balls--any one. What happens? All the balls move. The effect of your push seems to have been trans mitted almost without loss of time, from the first ball to the last.
Actually, though, the ball you pushed traveled a very small distance only and so did the ball next to it, and the ball next to that one.
The movement of current through a semiconductor is at an even slower pace than through a conductor, such as copper. In the case of p-type germanium we assumed that the holes would form an absolutely straight line so that the electrons could go hopping, also in a straight line, from one end of the germanium wire to the other. No such thing happens and we have no right to make such an assumption. The current might actually spread out and take a rather devious route. We've also enthused about the beauty of the orderly lattice structure of germanium. But nature isn't perfect by any means, and so some germanium crystals may not be as well constructed as others. B1untly, there may be imperfections and be cause of these imperfections the movement of the electrons may not be the smooth passage we imagine. Our meter in our series circuit won't reveal this to us, since it indicates just the average flow.
Other semiconductors We've been talking so steadily about germanium that we have perhaps created the false impression that this is the only semi conductor. The requirement is simple and any element that meets the requirement is eligible for joining the semiconductor club.
There aren't very many, however. Next to germanium, and quite possibly just as important, is silicon. Silicon has three electron rings (compared to four for germanium). The inner ring is the usual 2-electron orbit, the next ring has 8 electrons and the outer ring (the one that interests us) has 4.
Oddly enough, we have a somewhat unexpected member of the semiconductor family-lead. The ore of lead is lead sulphide or galena, and in the early days before vacuum tubes it was used as a semiconductor in crystal sets. We called them crystal sets since the galena looked like--and was--and is a crystalline substance.
And so, if you think semiconductors are new, the answer is that they have been with us since the very early days of radio.
Lead has no less than six electron rings: 2, 8, 18, 32, 18, 4. If you can think of all these electrons whirling in orbit around the lead nucleus, your imagination is in fine working order.
Gallium arsenide is another semiconductor material. Like lead sulphide, it is a compound. Gallium arsenide is being used in the manufacture of tunnel diodes. Although we call it a diode (Fig. 120), it can work as an oscillator, it can amplify, and is very useful as an electronic switch.
Gallium arsenide uses materials we have come to regard as acceptor and donor impurities. Gallium has 3 electrons in its outer most orbit; arsenic has 5. A semiconductor made of these elements is known as a III-V compound. Another type of semiconductor along these lines is gallium phosphide. This is also a 111-V crystal line compound semiconductor. Unlike germanium, some of these semiconductors will operate continuously at temperatures as high as 930°F. Thus, diodes produced from this material show promise for high-temperature operation, and as electroluminescent structures. The semiconductor itself is optically transparent. This makes it very useful in the basic study of semiconductors in general. For instance, it may actually be possible to see the differences caused by various degrees of doping and also the effects of various voltages.
The last of our semiconductors is tin, also rather surprising. But surprising or not, tin meets all the requirements. Tin is a sort of blue-white crystalline metal having five electron rings: 2, 8, 18, 18 and 4.
Fig. 120 . Tunnel diode is so small that tweezers are helpful in handling
it.
Looking Forward
The man who first realized that a slice of ham and a few slices of bread had unsuspected possibilities was a genius, for he in vented the ham sandwich. He's in the same class with those who looked at p-type germanium, at n-type germanium and then combined the two, for this led directly to the invention of the transistor. How this was done and what happens when we make this sort of electronic sandwich will give us mental food for thought in the next section.
QUIZ
1. What is an electron shell? How many electrons make a complete K shell?
2. Describe what is meant by n-type germanium.
3. What is a donor element? Name two and describe the effect of their diffusion into pure germanium.
4. What is meant by p-type germanium?
5. What is a lattice?
6. Can a copper wire or a bit of germanium accumulate electrons when connected to a source of voltage? Explain your answer.
7. Describe a conductor. An insulator. A semiconductor.
8. How does the movement of electrons through germanium compare to the movement of electrons through copper? Is there a difference?
9. Describe the formation of positive charges in germanium.
10. What is the direction of hole movement compared to electron movement?
11. What is a valence electron? How does it differ from other electrons?
12. What is a valence bond?
13. What is an acceptor element? Name two.
14. What difference, if any, exists between donor and acceptor elements?
15. What name or names do we use for the central portion of an atom?