AMAZON multi-meters discounts AMAZON oscilloscope discounts
Overview: What Is a Biosensor?
Biosensor = bio-receptor + transducer. A biosensor consists of two components: a bioreceptor and a transducer. The bioreceptor is a biomolecule that recognizes the target analyte, and the transducer converts the recognition event into a measurable signal. The uniqueness of a biosensor is that the two components are integrated into one single sensor. This combination enables one to measure the target analyte without using reagents. For example, the glucose concentration in a blood sample can be measured directly by a biosensor made speci fically for glucose measurement, by simply dipping the sensor in the sample. This is in contrast to the commonly performed assays, in which many sample preparation steps are necessary and each step may require a reagent to treat the sample. The simplicity and the speed of measurements that re quire no specialized laboratory skills are the main advantages of a biosensor.
AMAZON multi-meters discounts AMAZON oscilloscope discounts
Enzyme is a Bioreceptor. When we eat food such as hamburgers and french fries, it is broken down into small molecules in our body via many reaction steps (these breakdown reactions are called catabolism). These small molecules are then used to make the building blocks of our body, such as proteins (these synthesis reactions are called anabolism). Each of these catabolism and anabolism reactions (the combination is called metabolism) are catalyzed by a speci fic enzyme. Therefore, an enzyme is capable of recognizing a speci fic target molecule. This biorecognition capability of the enzyme is used in biosensors. Other biorecognizing molecules (= bioreceptors) include antibodies, nucleic acids, and receptors.
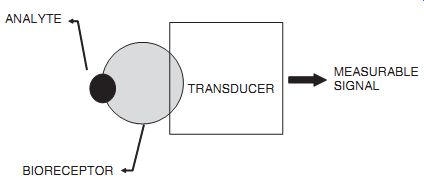
Biosensor configuration. TRANSDUCER ANALYTE; BIORECEPTOR MEASURABLE
SIGNAL
Immobilization of Bioreceptor. One major requirement for a biosensor is that the bioreceptor be immobilized in the vicinity of the transducer. The immobilization is done either by physical entrapment or chemical attachment. Chemical attachment often involves covalent bonding to transducer surface by suitable reagents. A comprehensive treatment of immobilization is available. It is to be noted that only minute quantities of bioreceptor molecules are needed, and they are used repeatedly for measurements.
Transducer. A transducer should be capable of converting the biorecognition event into a measurable signal. Typically, this is done by measuring the change that occurs in the bioreceptor reaction. For example, the enzyme glucose oxidase is used as a bioreceptor in a glucose biosensor that catalyzes the following reaction:
Gluconic acid + H2O2 Glucose + O Glucose Oxidase 2
To measure the glucose in aqueous solutions, three different transducers can be used:
1. An oxygen sensor that measures oxygen concentration, a result of glucose reaction
2. A pH sensor that measures the acid (gluconic acid), a reaction product of glucose
3. A peroxidase sensor that measures H2O2 concentration, a result of glucose reaction Note that an oxygen sensor is a transducer that converts oxygen concentration into electrical current. A pH sensor is a transducer that converts pH change into voltage change. Similarly, a peroxidase sensor is a transducer that converts peroxidase concentration into an electrical current. An excellent review of glucose sensing technologies was reported.
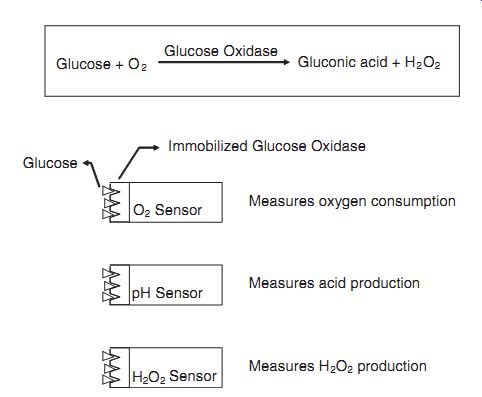
Three possible transducers for glucose measurement. O2 Sensor pH Sensor
H2O2 Glucose Oxidase Sensor Gluconic acid + H2O2 Glucose + O2 Glucose Immobilized
Glucose Oxidase Measures oxygen consumption Measures acid production Measures
H2O2 production Speci ficity of biosensor (TR: transducer). NO SIGNAL ; SIGNAL
; IMMOBILIZE
Biosensor Characteristics. Biosensors are characterized by eight parameters. These are: (1) Sensitivity is the response of the sensor to per unit change in analyte concentration. (2) Selectivity is the ability of the sensor to respond only to the target analyte. That is, lack of response to other interfering chemicals is the desired feature. (3) Range is the concentration range over which the sensitivity of the sensor is good. Sometimes this is called dynamic range or linearity. (4) Response time is the time required for the sensor to indicate 63% of its final response due to a step change in analyte concentration. (5) Reproducibility is the accuracy with which the sensor's out put can be obtained. (6) Detection limit is the lowest concentration of the analyte to which there is a measurable response. (7) Life time is the time period over which the sensor can be used without signi ficant deterioration in performance characteristics. (8) Stability characterizes the change in its baseline or sensitivity over a fixed period of time.
AMAZON multi-meters discounts AMAZON oscilloscope discounts
Considerations in Biosensor Development. Once a target analyte has been identi fied, the major tasks in developing a biosensor involve:
1. Selection of a suitable bioreceptor or a recognition molecule
2. Selection of a suitable immobilization method
3. Selection and design of a transducer that translates binding reaction into measurable signal
4. Design of biosensor considering measurement range, linearity, and minimization of interference, and enhancement of sensitivity
5. Packaging of the biosensor into a complete device
The first item above requires knowledge in biochemistry and biology, the second and third require knowledge in chemistry, electrochemistry and physics, and the fourth requires knowledge of kinetics and mass transfer. Once a biosensor has been de signed, it must be packaged for convenient manufacturing and use. The current trend is miniaturization and mass production. Modern IC (integrated circuit) fabrication technology and micromachining technology are used increasingly in fabricating bio sensors, as they reduce manufacturing costs. Therefore, an interdisciplinary research team, consisting of the various disciplines identi fied above, is essential for successful development of a biosensor.
Considerations for biosensor development.
- ¦ Selection of a suitable biorecognition entity
- ¦ Selection of chemical immobilization method
- ¦ Selection and design of a suitable transducer
- ¦ Designing of biosensor for measurement range, linearity, and minimization of interference
- ¦ Packaging of biosensor into a complete unit
Applications of Biosensors
Health Care
Measurement of Metabolites. The initial impetus for advancing sensor technology came from the health care area, where it is now generally recognized that measurements of blood chemistry are essential and allow a better estimation of the metabolic state of a patient. In intensive care units, for example, patients frequently show rapid variations in biochemical composition and levels that require urgent remedial action. Also, in less severe patient handling, more successful treatment can be achieved by obtaining instant assays. At present, available instant analyses are not extensive.
In practice, these assays are performed by analytical laboratories, where discrete samples are collected and shipped for analysis, frequently using the more traditional analytical techniques.
Market Potential. There is an increasing demand for inexpensive and reliable sensors for use in doctor's of fices, emergency rooms, and operating rooms. Ultimately, patients themselves should be able to use biosensors in the monitoring of a clinical condition, such as diabetes. It is probably true that the major biosensor market may be found where an immediate assay is required. If the costs of laboratory instrument maintenance are included, then low-cost biosensor devices can be desirable in the whole spectrum of analytical applications from hospital to home.
Diabetes. The "classic" and most widely explored example of closed-loop drug control is found in the development of an artificial pancreas. Diabetic patients have a relative or absolute lack of insulin, a polypeptide hormone produced by the beta cells of the pancreas, which is essential for glucose uptake. Lack of insulin secretion causes various metabolic abnormalities, including higher than normal blood glucose levels.
In patients who have lost insulin-secreting islets of Langerhan, insulin is supplied by subcutaneous injection. However, fine control is difficult to achieve and hyperglycemia is often encountered. Further, even hypoglycemia is sometimes induced, causing impaired consciousness and the serious long-term complications to tissue associated with this intermittent low glucose condition.
Insulin Therapy. Better methods for the treatment of insulin-dependent diabetes have been sought and infusion systems for continuous insulin delivery have been developed . However, regardless of the method of insulin therapy, its induction must be made in response to information on the current blood glucose levels in the patient.
Three schemes are possible, the first two dependent on discrete manual glucose measurement and the third a "closed-loop" system, where insulin delivery is controlled by the output of a glucose sensor which is integrated with the insulin in fuser. In the former case, glucose is estimated based on analysis of finger-prick blood samples with a colorimetric test strip or more recently with an amperometric pen size biosensor device by the patients themselves. Clearly, these diagnostic kits must be easily portable, simple to use and require minimal skill and easy interpretation. However, even with the ability to monitor current glucose levels, intensive conventional insulin therapy requires multiple daily injections. This open-loop approach does not anticipate insulin dosage due to changes in diet and exercise. For example, it was shown that administration of glucose by subcutaneous injection, 60 minutes before a meal provides the best glucose/insulin management.
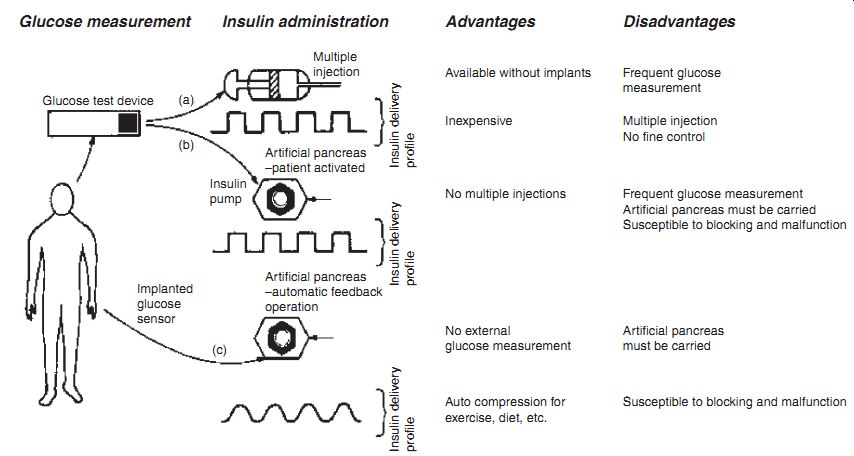
--- Schemes for insulin therapy.
Glucose measurement Insulin administration Advantages Disadvantages Available without implants Frequent glucose measurement Inexpensive Multiple injection No fine control No external glucose measurement Artificial pancreas must be carried Auto compression for exercise, diet, etc.
Insulin delivery profile Insulin delivery profile Insulin delivery profile Artificial pancreas -- patient activated; Artificial pancreas -- automatic feedback operation Insulin pump (a) (b); (c) Multiple injection Implanted glucose sensor Glucose test device Susceptible to blocking and malfunction; No multiple injections Frequent glucose measurement; Artificial pancreas must be carried; Susceptible to blocking and malfunction
---
Artificial Pancreas. The introduction of a closed-loop system, where integrated glucose measurements provide feedback control on a pre-programmed insulin ad ministration based on habitual requirements, would therefore relieve the patient of frequent assay requirements and, perhaps more desirably, frequent injections. Ultimately, the closed-loop system becomes an arti ficial pancreas, where the glycemic control is achieved through an implantable glucose sensor. A proposed implantable sensor is given. Clearly, the requirements for this sensor are very different from those for the discrete measurement kits. As summarized, the prolonged lifetime and biocompatibility represent the major requirements.
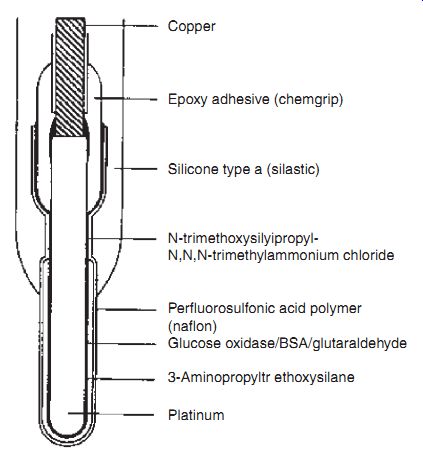
---- Cross-sectional view of an implantable glucose electrode in whole
blood.
Copper Epoxy adhesive (chemgrip); Silicone type a (silastic) N-trimethoxysilyipropyl N,N,N-trimethylammonium chloride Perfluorosulfonic acid polymer (naflon); Glucose oxidase/BSA/glutaraldehyde; 3-Aminopropyltr ethoxysilane Platinum
Requirements for an implantable glucose sensor.
- ¦ Linear in physiological range 0-20 mM
- ¦ Speci fic for glucose; not affected by changes in blood chemistry
- ¦ Biocompatible
- ¦ Small-causes minimal tissue damage during insertion
- ¦ Response time < 1 min
- ¦ Prolonged lifetime ~ in years
Industrial Process Control
Bioreactor Control. Bioreactors are used to cultivate recombinant cells for production of therapeutic proteins such as insulin. The productivity of such systems depends on bioreactor conditions. Real-time monitoring of carbon sources, dissolved oxygen and carbon dioxide, and products of metabolism in fermentation processes could lead to optimization giving increased product yields at decreased processing and material cost. While real-time monitoring with feedback control involving automated systems does exist, currently only a few common variables are measured on-line, (e.g., pH, temperature, CO2, O2) which are often only indirectly related to the culture activity under control. If cellular metabolic activity can be monitored in real-time using sensors, one can suitably alter the environmental variable to improve process productivity. The bene fit of closed-loop control of a cellular state are many, such as improved product yield and quality. It is the lack of sensors that limits the use of closed loop online control of cell culture and fermentation processes.
Military and Homeland Security Applications
The requirement for rapid analysis is also present in military applications. Recent military engagement in the Middle East has caused, rather rapidly, the deployment of field-usable sensors for chemical and biological warfare agents. Many of the sensors are small portable analytical kits that may be termed "dipsticks." While they are reasonably robust, their performance in the field has been reported to be variable. Thus, there is a large need for robust sensors that require no maintenance. Both contact as well as remote sensing for warfare agents are currently under development. Distributed sensors and systems for monitoring hazards due to terrorist activity are currently being developed under the auspices of the Department of Homeland Security funding.
Environmental Monitoring
Environmental Protection Agency (EPA) Air and Water Monitoring. EPA's Environmental Monitoring and Assessment Program (EMAP) was established to provide a comprehensive report card on the condition of the nation's ecological resources and to detect trends in the condition of those resources. EPA routinely monitors both water and air in urban and rural areas. In recent times the Department of Homeland Security has initiated efforts to monitor the environment in urban areas, particularly large population centers, for potential bioterrorism agents. The primary measurement media is water or air, but the variety of target analytes is vast. At sites of potential pollution or terrorist activity, it would be desirable to install on-line real-time monitoring and alarm, targeted at specific analytes. Common environmental analytes are biological oxygen demand (BOD), atmospheric acidity, and river water pH, detergent, herbicides, and fertilizer concentrations in drainage and river. The potential for biosensor technology for environmental monitoring is huge, and the potential impact is far-reaching. Although the principle of detection may be the same for a particular analyte, the actual technology platform used will be dependent on application. For example, a glucose sensor for on-line use in a fermenter has very different requirements from those used for monitoring glucose concentration in diabetic patients. Customization to meet application needs is often a very important part of technology development.
Origin of Biosensors
Enzyme Electrode. The biosensor was first described by Clark and Lyons (1962), when the term enzyme-electrode was introduced. In this first enzyme electrode, an oxido-reductase enzyme, glucose oxidase, was held next to a platinum electrode in a membrane sandwich. The platinum anode polarized at +0.6 V responded to the peroxide produced by the enzyme reaction with substrate. The primary target substrate for this system was glucose:
Gluconic acid + H2O2 Glucose + O Glucose Oxidase 2
and led to the development of the first glucose analyzer for the measurement of glucose in whole blood. This Yellow Springs Instrument (Model YSI 23) appeared on the market in 1974, and the same technique as employed here has been applied to many other oxygen-mediated oxido-reductase enzyme systems.
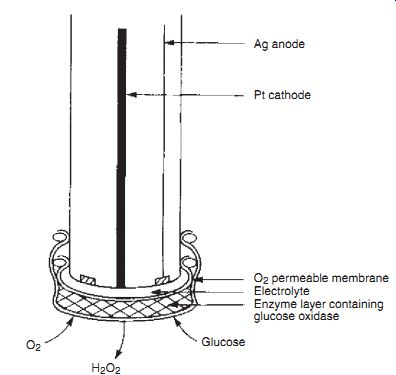
The Clark Enzyme Electrode. Ag anode Pt cathode
O2 permeable membrane Electrolyte Enzyme layer containing glucose oxidase
Glucose H2O2, O2
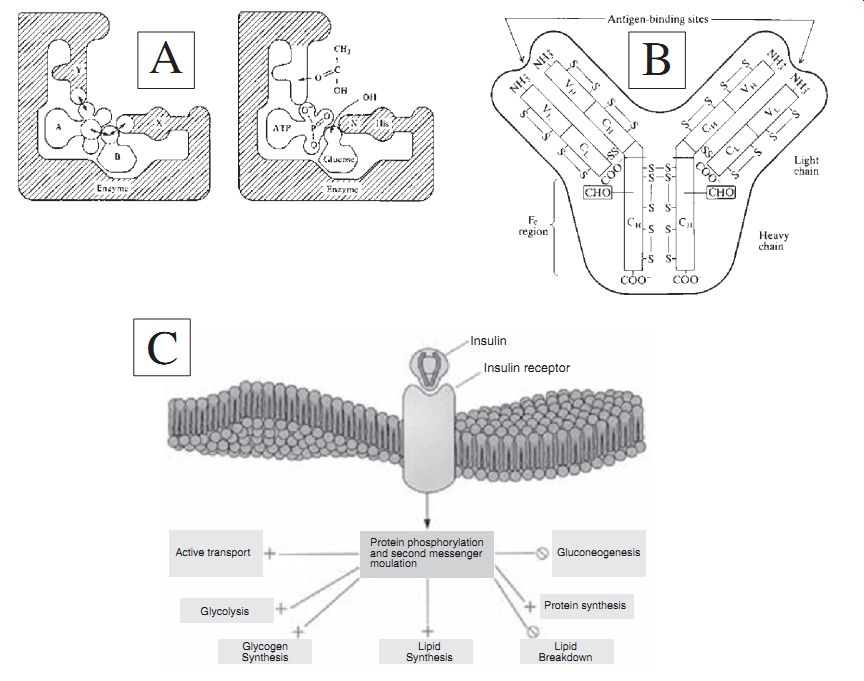
Bioreceptor molecules used for biosensor applications: (A) Enzyme; (B)
Antibody; (C) Protein receptor.
Receptor Protein. Receptor proteins have speci fic af finity for biologically active compounds. These proteins are mostly bound to membrane. There are hormone receptors, taste receptors, olfactory receptors for smelling, photoreceptors for eyes, and others. Receptor proteins activate opening and closing of membrane channels for transport of speci fic metabolites. They also play a key role in transducing intracellular messages for responsive action. Since the receptor proteins recognize speci fic biological entities, they are often used to measure target analytes. For example, death receptors on a cell surface transmit apoptosis signals initiated by speci fic ligands. They play an important role in apoptosis and can activate caspase cascade within seconds of ligand binding. Thus, when a death receptor is a sensing entity, one can potentially mea sure the presence of apoptotic-inducing chemicals in the environment.
Other Approaches. In principle, any bio-molecules and molecular assemblies that have the capability of recognizing a target analyte can be used as a bioreceptor. In fact, membrane slices or whole cells have been used in biosensors.
--- summarizes possible bioreceptors that can be utilized in a biosensor. Note that the bioreceptors require a suitable environment for maintaining their structural integrity and biorecognition activity. These requirements are described, along with the type of signal generated as a result of the biorecognition activity. The transducer in a biosensor should be responsive to this biochemical activity.
Bio-layer; Type Main needs for structural integrity; Typical signal generated
End of Organ (e.g., Olfactory)
Intact tissue architecture; Action potential
Tissue
Nutrient/O2 supply; Metabolic end product; Whole cell
Cell organelle (e.g., mitochondorion)
Osmotic/pH stability; Product of electron chain
Complexity hierarchy; Biomembrane (e.g., receptor)
Mechanic protection; Released contents
Enzyme pH/electrolyte stability; Reaction product
Antibody pH stability; Antigen uptake/ mass change
Ionophore; Adequate retention EMF/absorbance change (chromionphore)
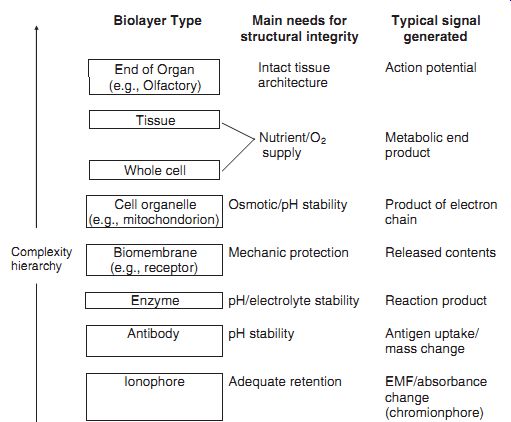
Possible bioreceptor molecules and molecular assemblies for biosensor
applications; their requirements for structural integrity and signals
generated.
5. Transduction Mechanisms in Biosensors
Conventional Transducers. The majority of biosensors in use today use three types of transducers for converting the action of the bioreceptor molecule into a measurable signal. These are: amperometry based on H2O2 or O2 measurement; potentiometry based on pH or p_Ion measurement; and photometry utilizing optical fibers.
Biorecognition reactions often generate chemical species that can be measured by electrochemical methods. In these, typically the reaction product is H2O2 (or the reactant is O2) which can be measured by a pair of electrodes. When a suitable voltage is impressed on one of the electrodes against a reference electrode (typically Ag/ AgCl or Calomel), the target species (H2O2 or O2) is reduced at the electrode and this generates electrical current (hence the name amperometry). In potentiometry, a glass membrane or a polymeric membrane electrode is used for measuring the membrane potential (hence the name potentiometry) resulting from the difference in the concentrations of H+ or other positive ions across the membrane. In photometry, the light from an indicator molecule is the measured signal. In this method, one of the reactants or products of the biorecognition reaction results in colorimetric, fluorescent or luminescent changes that are measured using photodetectors. Usually, an optical fiber is used for guiding the light signals from the source to the detector. Adaptation and exploitation of these three routes, (amperometric, potentiometric and photometric), where user acceptability is already established, has been an obvious approach to the development of reagentless biosensor devices.
Piezoelectric Transducers. The transducer of a biosensor is not restricted to the three described above. In principle, any variable that is affected by the biorecognition reaction can be used to generate the transduced signal. The piezoelectric materials and surface acoustic wave devices offer a surface that is sensitive to changes in mass.
These transducers have been used where the biorecognition reaction causes a change in mass. For example, piezoelectric silicon crystals-called quartz crystal microbalance (QCM)-have been used to measure very small mass changes in the order of picograms. QCM with immobilized antibody to pathogens have been successfully used to measure the presence of pathogens in aqueous samples. Piezoelectrically driven cantilevers have also been used to measure adsorption of very minute quantities of biochemicals. Conductimetric Transducers. Monitoring solution conductance was originally applied as a method of determining reaction rates. The technique involves the measurement of changes in conductance due to the migration of ions. Many enzyme linked reactions result in a change in total ion concentration and this would imply that they are suitable for conductimetric biosensors.
Electrical Capacitance as Transducer. When the biorecognition reaction causes a change in the dielectric measurement constant of the medium in the vicinity of the bioreceptor, the capacitance measurement method can be used as a transducer.
Antigen-antibody reaction is a good example. Suppose antibody molecules are immobilized between two metal electrodes of known area. When antigen is added and binds with the antibody, the dielectric constant of the medium between the two electrodes is expected to change signi ficantly. This change translates into a change in capacitance.
Thermometric Transducer. All chemical reactions are accompanied by the absorption (endothermic) or evolution (exothermic) of heat. Measurements of ?H, the enthalpy of reaction at different temperatures, allows one to calculate ?S (entropy) and ?G (Gibbs free energy) for a reaction and therefore collect basic thermodynamic data. The hydrolysis of ATP for example is exothermic:
ATP4- + H2O~ADP3- + HPO4- + H+ ; ?H298 = -22.2 kJ (pH 7)
... or the immunoreaction between anti-HSA and its antigen HSA yields -30.5 kJ/mol.
For this latter reaction, the total increase in temperature for 1 mmol of antibody is of the order of 10^-5 K, but many enzyme-catalyzed reactions have greater ?H, and produce more easily measurable changes in temperature.
Enzyme Thermistor. For a biosensor device, the biorecognition compound must be immobilized on a temperature-sensing element capable of detecting very small temperature changes. The major initiative in this area has come from the Mosbach group at the University of Lund. Initially, they immobilized glucose oxidase or penicillinase in a small column, so that temperature changes in the column ef fluent were monitored by thermistors to give an enzyme thermistor sensitive to glucose and penicillin, respectively. They have also applied the technique to other substrates and to immunoassay using an enzyme-labeled antigen.
FET as a Transducer. As advances are made in biosensors, a need has developed for miniaturization and mass production. Field effect transistors (FET) used extensively in the semiconductor industry in memory chips and logic chips respond to changes in electric field (in front of the "gate" of the FET). An FET is thus capable of detecting changes in ion concentration when the gate is exposed to a solution that contains ions. Therefore, pH and ion concentration can be measured with an FET. The advantage of this transducer is that it can be incorporated directly into the electronic signal processing circuitry. In fact, a pen-size FET-based pH sensor is being marketed commercially.
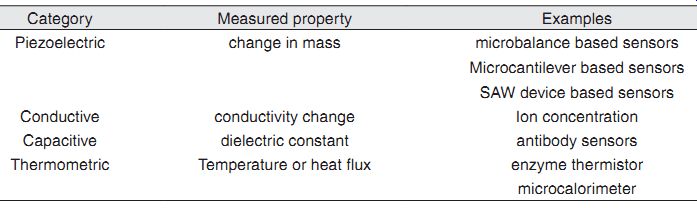
-----: Other transducers used in biosensors.
Category Measured property Examples Piezoelectric change in mass microbalance based sensors Microcantilever based sensors SAW device based sensors Conductive conductivity change Ion concentration Capacitive dielectric constant antibody sensors Thermometric Temperature or heat flux enzyme thermistor micro-calorimeter
6. Application Range of Biosensors
Current Status. Since the development of Clark's glucose sensor for monitoring fermentation processes, many enzyme electrodes have been developed based on amperometry, potentiometry, and photometry. A sample of these biosensors is summarized. The term "optode" is used for sensors utilizing optical fiber for light signal transmission. Note that the bioreceptors used are all enzymes except the antibody sensor.
==
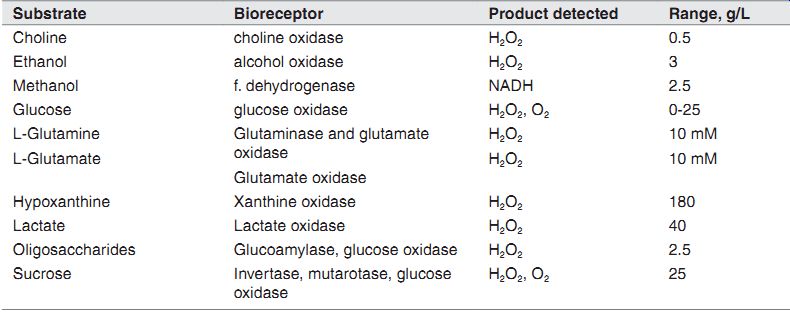
Amperometric biosensors.
Substrate Bioreceptor Product detected Range, g/L Choline choline oxidase H2O2 0.5 Ethanol alcohol oxidase H2O2 3 Methanol f. dehydrogenase NADH 2.5 Glucose glucose oxidase H2O2, O2 0-25 L-Glutamine L-Glutamate Glutaminase and glutamate oxidase Glutamate oxidase H2O2 H2O2 10 mM 10 mM Hypoxanthine Xanthine oxidase H2O2 180 Lactate Lactate oxidase H2O2 40 Oligosaccharides Glucoamylase, glucose oxidase H2O2 2.5 Sucrose Invertase, mutarotase, glucose oxidase H2O2, O2 25
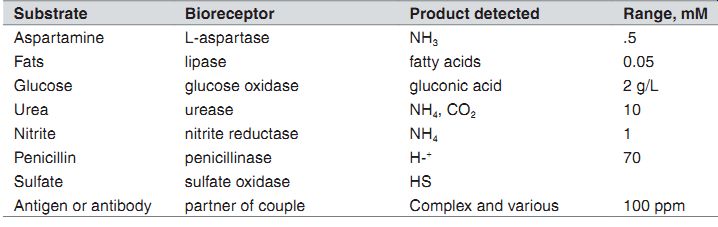
-----: Potentiometric Biosensors.
Substrate Bioreceptor Product detected Range, mM Aspartamine L-aspartase NH3 .5 Fats lipase fatty acids 0.05 Glucose glucose oxidase gluconic acid 2 g/L Urea urease NH4, CO2 10 Nitrite nitrite reductase NH4 1 Penicillin penicillinase H-+ 70 Sulfate sulfate oxidase HS Antigen or antibody partner of couple Complex and various 100 ppm

-----: Enzyme sensors based on optodes
Substrate Bioreceptor; Product detected; Range, mM Ethanol alcohol dehydrogenase NADH 1 Glucose glucose oxidase O2 20 Urease urease ammonia 3 Lactate lactate monooxygenase pyruvate 1 Penicillin penicillinase penicillinic acid 10
==
Products Detected. As is noted, the analyte is indirectly measured by measuring the product of a reaction due to the enzyme. It is oxygen that is actually measured, and its concentration is directly proportional to the analyte concentration. For amperometry, the majority is H2O2 (with the exception of NADH), which is the common product for oxido-reductase enzymes. For potentiometric biosensors, the majority is acid which is detected by a pH sensor. Infermentor and cell culture reactors, products of metabolism CO2 and NH3 are indirectly detected by measuring the change in pH.
Biosensor Configurations. When bioreceptor molecules are combined with a suitable transducer, a biosensor is made. Figure 6.1 shows various biosensor configurations. Note that the bioreceptor molecules are immobilized in a suitable matrix to form a bioactive layer, which is then placed in the immediate vicinity of a transducer. The transducer's ion-selective electrode and FET belong to the potentiometric transducer category; the coated wire belongs to the amperometric sensor category; the surface plasmon detector and the surface acoustic wave detector belong to the piezo transducer category. The materials of construction for the transducers are also given in the figure.
==
---
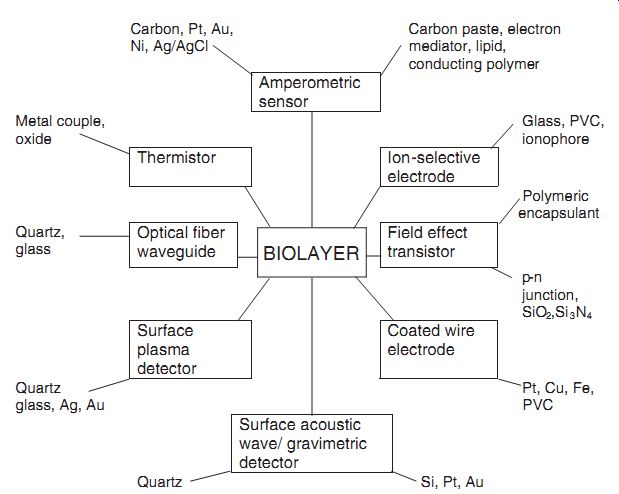
---: Various biosensor configurations.
Carbon, Pt, Au, Ni, Ag/AgCl
Carbon paste, electron mediator, lipid, conducting polymer
Amperometric sensor
Metal couple, oxide
Glass, PVC, ionophore
Thermistor Ion-selective electrode
Polymeric encapsulant
Quartz, glass
Optical fiber waveguide
BIOLAYER
Field effect transistor p-n junction, SiO2,Si3N4 Surface plasma detector
Coated wire electrode
Quartz glass, Ag, Au Pt, Cu, Fe, PVC
Surface acoustic wave/ gravimetric detector
Quartz Si, Pt, Au
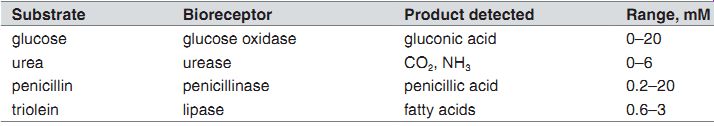
Biosensors based on FET (pH).
Substrate Bioreceptor
Product detected Range, mM glucose glucose oxidase gluconic acid 0-20 urea urease CO2, NH3 0-6 penicillin penicillinase penicillic acid 0.2-20 triolein lipase fatty acids 0.6-3
-----
Discriminative Membranes. Membranes are one of the most essential components of a biosensor. They are used for (1) barriers to non-analyte molecules, (2) protection of enzyme-immobilized membrane, thus preventing fouling; and (3) controlling the operating range of the biosensor. When a small molecule is the analyte, macromolecules such as proteins can be prevented from entering the active sensing area by using a small pore membrane. Note that proteins adsorb readily on most surfaces and thus foul sensing surfaces. The transport of charged molecules can be modulated by placing ion selective membranes. A combination of various discriminative membranes can be used for blocking the passage of different interfering molecules. It is to be noted that the use of a discriminative membrane increases time lag as it introduces diffusive transport resistance, and thus judicious choice of its thickness is essential for proper functioning. A summary is given.
-----
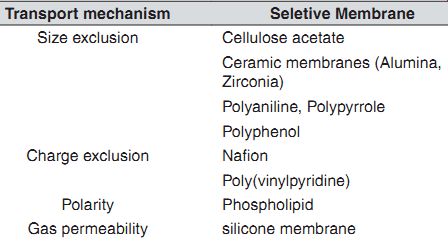
Discriminative coatings for amperometric biosensors.
Transport mechanism Seletive Membrane Size exclusion Cellulose acetate Ceramic membranes (Alumina, Zirconia) Polyaniline, Polypyrrole Polyphenol Charge exclusion Na flon Poly(vinylpyridine) Polarity Phospholipid Gas permeability silicone membrane
-------
Sensitivity Requirements. The range and type of analytes fall into wide and varied values and thus cannot be considered using a single criterion. A particular application dictates the concentration range needed. It is often determined on the basis of expected target concentration range in samples. For example, a metabolite's concentration range is often in the uM range, whereas hormones are in nM range. Viruses and pathogens are found in 10-10,000 per mL. This vast range of concentrations is summarized in Figure 6.2. It is thus clear that often a varied approach is needed for a sensor designed for a metabolite compared with measuring tumor antigens.
Evolution of Biosensors. Biosensors can be classi fied into three generations according to the degree of integration of the separate components--i.e. the method of attachment of the biorecognition or bioreceptor molecule to the base transducer element. In the first generation, the bioreceptor is physically entrapped in the vicinity of the base sensor be hind a discriminating membrane such as a dialysis membrane. In subsequent generations, immobilization is achieved via covalent bonds at a suitably modi fied transducer interface or by incorporation into a polymer matrix at the transduction surface. In the second generation, the individual components remain essentially distinct (e.g., control electronics-electrode-biomolecule), while in the third generation the bioreceptor molecule becomes an integral part of the base sensing element. While these de finitions were probably intended for enzyme electrode systems, similar classifications appropriate to biosensors in general can be made. It is in the second and third generations of these families that the major development effort can now be seen.
7. Future Prospects
In recent years the emerging area of nanotechnology has produced very interesting materials, some of which provide opportunities for new sensing transduction technologies useful for biosensor development. In addition, use of self-assembly techniques and nano-electromechanical systems have produced new laboratory sensing methodologies. Some of these approaches, while not robust for common analytical instrumentation or field use, will emerge in future as practical sensors. The short discussion given below is to provide a snapshot of emerging methods.
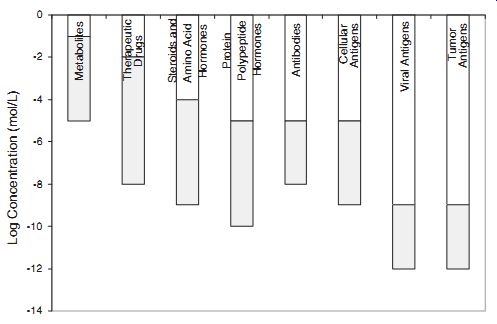
Detection ranges required for some clinically important analytes.
Metabolites Therapeutic Drugs Steroids and Amino Acid Hormones Protein Polypeptide Hormones Antibodies Cellular Antigens Viral Antigens Tumor Antigens Log Concentration (mol/L)
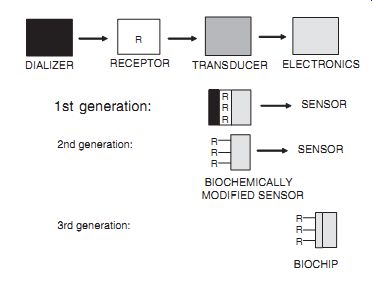
--- Three biosensor generations (R: Bioreceptor
molecule). DIALIZER RECEPTOR TRANSDUCER ELECTRONICS SENSOR 1st generation:
SENSOR 2nd generation: BIOCHEMICALLY MODIFIED SENSOR 3rd generation: BIOCHIP
Mass change sensors rely on changes in resonant frequency, as natural frequency depends on mass of oscillating mass. In this category, the quartz crystal microbalance (QCM) or thickness shear mode oscillator has been extensively used for detecting the presence of antigens by modifying the surface of QCM with an antibody speci fic to the target antigen. The same principle has been attempted in other forms of oscillating devices, such as silicon microcantilevers, piezoelectric-excited microcantilevers, surface acoustic wave (SAW) sensors and others.
In a SAW device, electrodes are on the same side of the crystal and interdigital transducers act as a transmitter and receiver to excite surface waves that travel across the crystal face. The changes to the wave caused by target antigen binding to the surface is con fined to the crystal face, and is measured. SAW sensors are considerably more sensitive than QCM, but when aqueous phase is present on the surface, the signals are considerably attenuated. On the other hand, when no liquid solution is in contact, it provides very sensitive measurements for gas phase composition.
Raman spectroscopy is a useful tool for analysis because of its excellent chemical group identi fication capability; however, its limitation is low sensitivity. Recent observation that Raman scattering ef ficiency can be enhanced by many orders of magnitude when the analyte is adsorbed or near a gold or silver surface has made this technique a very powerful sensing methodology. This modi fied technique, known as surface enhanced Raman scattering (SERS), has been shown to be suitable in a laboratory setting to observe DNA hybridization. Thus, single strands of DNA fragments can be labeled to SERS probes. The resulting SERG probes may be used to identify genes or detect bacterial and viral components. A further improvement can be achieved by using peptide nucleic acid (PNA), which was originally developed as a gene-targeting drug. PNA has demonstrated remarkable hybridization properties towards complementary oligonucleotides. Consequently, biosensors based on replacement of the DNA recognition layer with a PNA one, offer signi ficantly improved distinction between closely related DNA sequences, as well as several other attractive advantages.
There has also been considerable interest in biophotonic sensors and DNA sensors. For example, resonance enhancement due to gold nano-particles bound to recognition molecules has been shown to be effective in biophotonics. The advantage of photonics is the ability to measure without contacting the sample. Use of fiber optics and its variants have provided a rich source of transducing elements. In these cases, the surface of the fiber (glass) is derivatized with an amine group and then covalently linked to a bioreceptor via carboxylic group. When an analyte binds, the light transmission characteristics are altered and that is measured. Due to availability of inexpensive monochromic light sources (light emitting diodes) and inexpensive light sensing devices (photo diodes), biophotonic devices offer a relatively inexpensive sensing platform.
In this section an overview of biosensors was presented, various elements of biosensors were described, and a brief review of bioreceptors and transduction mechanisms were provided.
NEXT: Chemical Sensors
PREV: