AMAZON multi-meters discounts AMAZON oscilloscope discounts
Chemical sensors are used to detect the presence of specific chemical compounds or elements, and their concentrations. This section covers some basic concepts for sensing of chemical quantities and some important applications.
Technology Fundamentals
The Nose
The human nasal sensing apparatus contains a remarkably flexible and sensitive detection capability. Humans are capable of detecting and distinguishing thousands of different smells with almost instantaneous recognition. Odor detection is made very complicated because of the lack of uniqueness in the chemical basis of most smells. There is no "garlic molecule" that is distinct from the "enchilada molecule," yet a person can easily distinguish these smells.
AMAZON multi-meters discounts AMAZON oscilloscope discounts
All biological odor detection systems are based on a fairly small number of distinguishable sensors. The smell recognition system is based on pattern-matching of the response of the different chemical sensors in the nose to various odors. Garlic and enchiladas produce a slightly different collective response in the entire set of sensors in your nose, and your brain has stored an extensive collection of these patterns which are used for comparisons. Psychologists have found that these odor patterns can be among the strongest of memories, and smells are often used to aid in the reconstruction of memories.
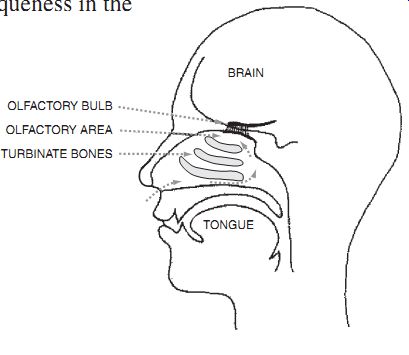
Human nose. OLFACTORY BULB; OLFACTORY AREA; TURBINATE BONES; BRAIN;
TONGUE
Chemical sensing system designers need to draw lessons from these biological systems. An important lesson is that a multifunctional system will probably need to use a small set of distinct sensors and a pattern-matching algorithm to identify odors accurately.
Detectors of Particular Molecules
If a chemical sensing application requires detection of a particular molecule, several techniques are available. These techniques are based on the unique properties of particular molecules.
One set of properties is associated with the vibrational and rotational modes of molecules. The exact energies of these modes are generally unique to a particular molecule, and may be used for identi fication purposes. Most vibrations and rotations are "optically active," meaning that they may be excited by absorption of a photon, or may relax by emission of a photon. These photon absorptions are generally most likely to occur in the infrared, so infrared spectroscopy is a generally useful way to identify molecules.
For example, CO is a very simple molecule (visualize two balls and a spring), capable of oscillating at a single frequency (visualize them bouncing together and away) and rotating about two axes, both perpendicular to the line connecting the atoms. In quantum mechanics, a vibration is represented as a single frequency-the molecule may be in the ground state, or in any of a number of excited states, each of which is separated by the energy of the mode: hw/(2p). Quantum mechanics includes "Selection Rules" which strongly favor relaxation a single step of hw/(2p) at a time. This feature shows up in the infrared spectrum as a single absorption.
In the spectrum of the absorption of CO, shown, we see a pair of absorption peaks. This peak splitting is due to the fact that carbon exists in isotopes which have atomic mass of 12 or 13. The additional mass of the C13 reduces the vibrational energy simply because it lowers the resonant frequency w k m = ( ) . In addition to vibrations, molecules also can have rotational energy. In the spectrum of NH3 (ammonia), the rotational spectrum contributes a series of closely spaced lines centered about the vibrational peaks. These excitations involve the absorption of a photon and the change of both the vibrational and rotational energy.
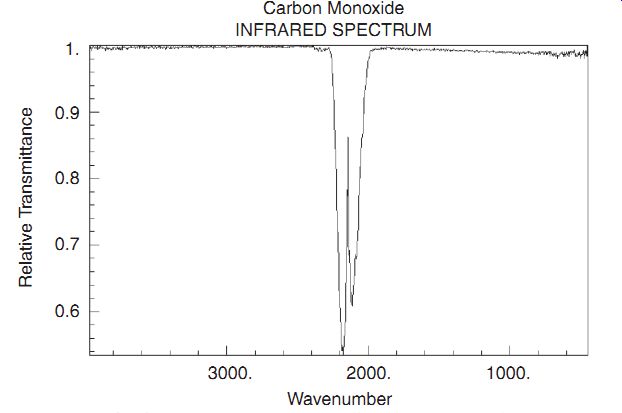
CO absorption spectrum. Carbon Monoxide; INFRARED SPECTRUM
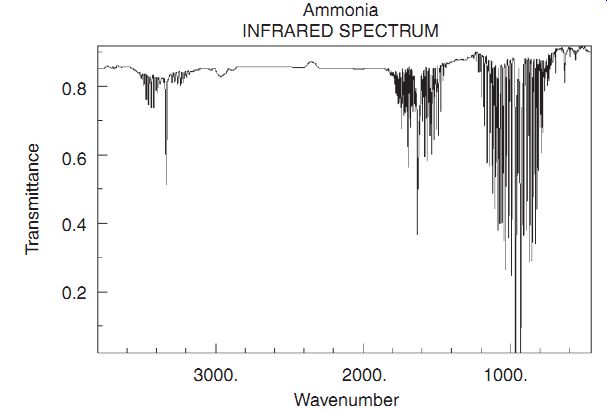
NH3 spectrum. Ammonia; INFRARED SPECTRUM Wavenumber
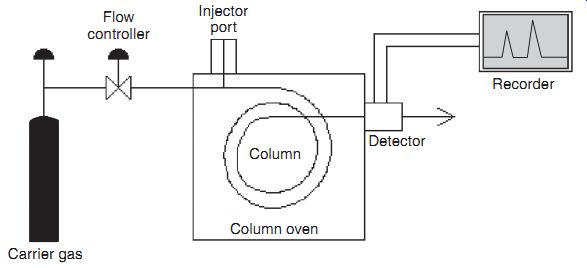
Gas chromatograph. Column oven; Carrier gas; Flow controller; Injector port;
Recorder; Detector; Column
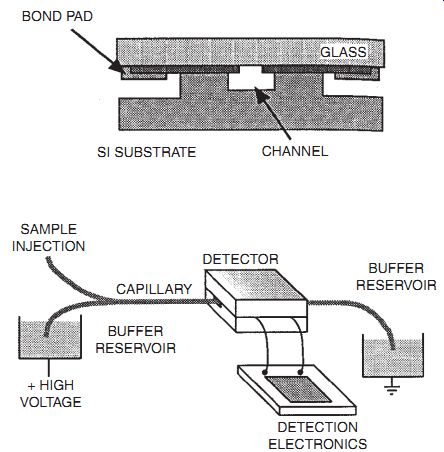
Electrophoresis instrument and its data.
Here, there is a magnetic field, which exerts a Lorentz force on each molecule, tending to deflect the trajectory of the molecules. The amount of deflection depends on the velocity and on the mass, and therefore acts to separate the molecules by their mass. A series of detectors acts to record the molecule concentration vs. deflection angle, and the output can be displayed as a mass spectrum. One important drawback to such an instrument is that the molecules must travel their entire path without scattering from other molecules. In air at atmospheric conditions, the aver age distance between collisions is 1 micron. Since typical mass spectrometers need 10-100 cm of trajectory, the pressure must be 6-9 orders of magnitude lower than atmospheric pressure. Therefore, mass spectrometers generally require vacuum pumps.
In addition to the magnetic mass spectrometers, there are spectrometers based on oscillating electric fields (quadruple analyzers), and on the mass-velocity relationship for particles with the same kinetic energy (time of flight). These spectrometers all have similar requirements on pressure.
Finally, a series of chromatography instruments are available. In these instruments, the varying molecular diffusivities are used to detect specific molecules. A typical gas chromatograph is schematically shown.
AMAZON multi-meters discounts AMAZON oscilloscope discounts
Here, a sample is added to a pressurized carrier gas, and forced to diffuse through a "column," which is essentially a very long narrow tube. The components of the sample diffuse at different rates through the column, and the detector at the end re cords a signal-vs.-time trace which contains peaks that may be identi fied as belonging to a specific sample. Chromatography has been in use for a long time, and is generally carried out in table-top instruments costing $10,000 to $100,000. Miniaturization of these instruments is currently the subject of much research in industry and academia.
Microfabrication techniques can be used to manufacture the column and the detector area.
An example is shown from research in the Kovacs group at Stanford, in the form of a capillary electrophoresis instrument shown.
In electrophoresis, an electric voltage bias is applied to the column, and the carrier fluid is conductive. Therefore, there is a steady flow of carrier fluid ions through the column, which sweeps the sample along with it. Again, the diffusivity differences for different sample components causes the sample to be spread out into a spectrum, and the resulting output trace may be analyzed to identify the components and quantify their concentrations.
Electrochemical Detection Techniques
The human body also does a great many chemical measurements on body fluids. The basic principles behind such measurements are discussed in the following paragraphs.
If a semi-permeable membrane separates two solutions, it may be possible for one component of the solutions to diffuse through from one side to the other. This notion of "semi-permeable membranes" may sound fanciful, but there are many biological examples of cell membranes that pass only a few nutrients.
In H2O solutions, many atoms and molecules exist in a charged state (Na generally has a +1 charge, for example), so the diffusion of these molecules also represents a diffusion of charge.
Now, when the two solutions are introduced, it is possible for a difference to exist in the concentration of the mobile ion on the two sides of the membrane. In general, one side is a reference sample, and the other side is a sample to be tested. Ions from each side begin immediately to diffuse through the membrane.
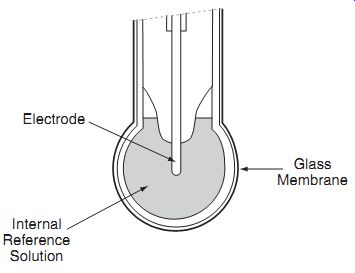
Fig. 1.7 pH electrode. Internal; Reference; Solution; Electrode; Glass; Membrane
Fig. 1.7 shows a glass membrane separating a sample solution (on the outside) from a reference solution (inside), and electrodes to measure the potential difference.
If the concentrations are different on the two sides of the membrane, the amount of diffusion will be different, leading to a net diffusion.
Since there is also a charge associated with these ions, there is a current. Very quickly, the motion of charge across the membrane causes the formation of an electric field, which opposes the flow of ions. An equilibrium is established when the electric field is large enough to overcome the concentration difference and the diffusion rates become balanced.
At this point the potential difference across the membrane is absolutely related to the concentration ratio. The relationship is given in introductory chemistry textbooks as the Nernst equation:
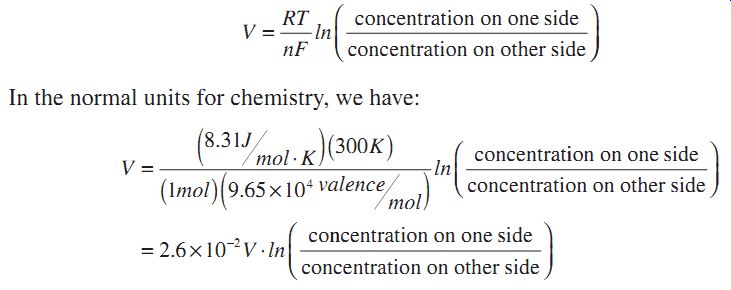
So, if there is a concentration ratio of 2, there will be a potential difference of about 20 mV. Such a potential difference is very easily measured.
In most actual situations, the thickness of the membrane is not too thin, so the potential difference builds up before there has been much actual transfer of concentration. This is important, because otherwise the required diffusion would alter the concentrations.
2. Applications
Automotive
An important automotive application for such a sensor is the automotive exhaust oxy gen sensor. In an automobile, fuel and air are mixed and introduced into the cylinder for combustion. The mixture can contain too much fuel (rich) or too much air (lean). In older cars, this mixture was adjusted manually. Optimal performance was obtained by a slightly rich mixture, because this maximizes the compression ratio.
However, the EPA began to monitor automotive emissions, and determined that a rich mixture also leaves a large amount of undesirable hydrocarbons in the exhaust, which foul the air and have undesirable consequences for the ozone. In the 70s and 80s, it became required to have a control system that maintained the fuel-air ratio at the precise value needed for optimal combustion.
This legislative mandate was made possible by a very fortunate coincidence. Accurate measurement of the fuel and air input to the mixing chamber would be very expensive. However, after combustion, measurements can be carried out in the exhaust.
During combustion of a rich mixture, very nearly all of the oxygen is consumed. During combustion of a lean mixture, the oxygen concentration is nearly the same as in the atmosphere (1-10%). So, it is possible to determine the state of the mixture very accurately by doing a simple measurement of the oxygen in the exhaust.
In a good automotive system, the concentration ratio for rich and lean may be different by 10-20 orders of magnitude. An electrochemical sensor that compared the oxygen concentration in the exhaust to that in the surrounding air would produce volt ages near 1 V or near 0 V for those two conditions-easily distinguished by an engine controller.
O2 can diffuse in ceramic, so oxygen sensors can be made by producing a ceramic "nipple," whose inside surface is coated with a metal electrode. The potential between the inside surface and the surface exposed to the exhaust is measured and used to control the fuel/air mixture. ---- shows the arrangement of electrodes.
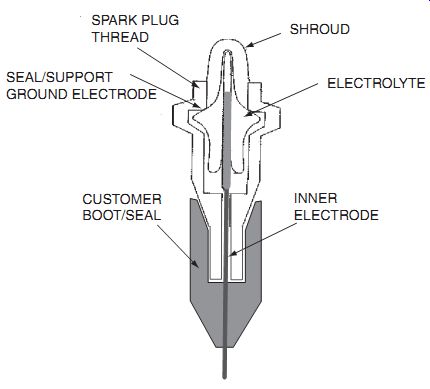
Fig. 2.1 O2 sensor. SHROUD; ELECTROLYTE; INNER ELECTRODE; SPARK PLUG THREAD;
SEAL/SUPPORT GROUND ELECTRODE; CUSTOMER BOOT/SEAL.
However, this basic technology has some undesirable characteristics. The sensor operates differently cold, and so cars are not optimized until the emissions system warms up. At present, most emissions from an automobile take place in the first few minutes after starting. In coming years, you should expect to see the EPA mandate heaters for more rapid warming of these sensors.
Other Miscellaneous Chemical Sensing Techniques and Applications
The measurement of impurities in water is becoming very important. Before using more expensive detection techniques, it is often easiest to simply measure the conductivity directly. Water's conductivity varies by several orders of magnitude as it varies from ultrapure to ordinary tap water. As for any other resistance measurement, a simple resistance bridge is often suf ficient, as shown.
If it is important to avoid direct electrical contact with the fluid because of chemical effects, the noncontact approach shown will work. In this case, a measurement like the metal detector measurements is carried out. The conductivity of the fluid decreases the mutual inductance between a pair of coils wound around the fluid conduit. The disadvantage of this approach is that it is hard to detect the fluid conductivity if the pipes are themselves conductive.
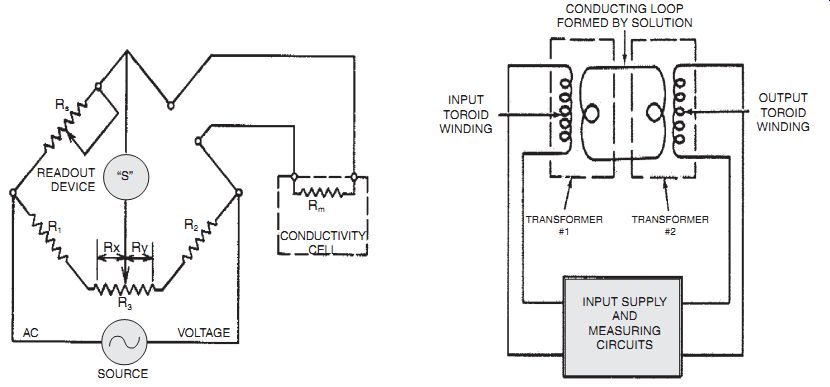
Fig. 2.2 Conductivity measuring circuits. Noncontact conductivity measurement.
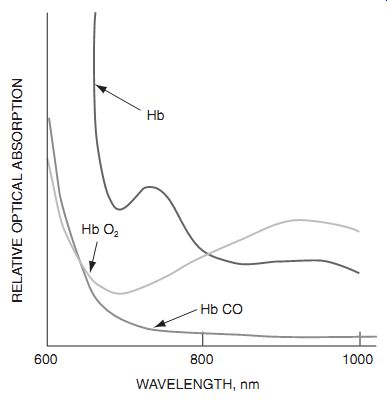
Fig. 2.3 Optical absorption spectra.
CHEMFETs
Another method for chemical detection in solutions or in gas relies on the charge transfer that can occur during a chemical reaction on a surface. In these devices, the surface of interest is a metal electrode which is actually the exposed gate of a field effect transistor (FET). Since the conduction from source to drain in a FET is modi fied by charge on the gate, this device can be a remarkably sensitive detector of certain absorbed species. These sensors are called CHEMFETs.
Of course, to build a selective detector, it is necessary to select a metal electrode that allows only one chemical reaction to occur. Simple metals are not very selective, so simple CHEMFETs suffer from a lack of selectivity-meaning that they respond to many different chemical species. One way to improve the selectivity of such a sensor is to follow a biological example, and to coat the electrode with molecules that are in deed very selective. Antibodies are molecules that tend to react only with a particular (virus) molecule, and are more chemically selective than any simple metal electrode.
Finally, there are a number of medical applications that rely on detection of oxygen in the bloodstream. Unfortunately, the bloodstream is a dif ficult place to work because white blood cells interpret the presence of almost any foreign matter as an invading organism, and tend to form scabs on all surfaces of such objects.
Blood does exhibit a detectable change in color upon the absorption of oxygen, and blood oxygen may be crudely measured by looking at blood color. For example, a sensor that measures blood r eflectivity at 700 nm and at 800 nm ought to be able to measure the blood oxygen content very accurately.
The measurement at 800 nm is used to cancel out effects of scab overcoating.
One possible implementation is a fiber-op tic system that transmits light of two colors (700 and 800 nm), and senses the r eflected light intensity as a measure of blood oxy gen. Such a system is often used during surgical procedures but is not typically used for long-term implants.
One device uses an LED emitter and a pair of detectors, each mounted looking out the side of a 1-mm thick catheter. The emitter and detector are separated by a few millimeters, so this instrument samples to a depth of a few millimeters, and is not badly affected by an overcoating of "scab." This same technique can be applied to the measurement of skin color.
Summary
There are many different applications for chemical sensors, and many techniques which can be applied for any given application. In general, chemical sensing devices do not compare favorably to biologically developed detectors. Devices generally suffer from a lack of sensitivity, selectivity, and speed. For some applications, the signals of interest are large and easy to detect. For others, it is very tough going. Research can be expected to grow in detection of toxins in groundwater, vehicle emissions, biotoxins in public settings, and a large variety of chemicals in manufacturing process control.
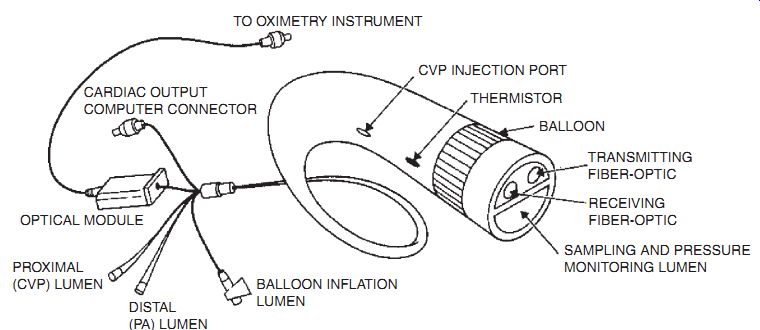
Fig. 2.4 Opticath catheter.
NEXT: Capacitive and Inductive Displacement Sensors
PREV: Biosensors