(source: Electronics World, Apr. 1968)

By HOWARD J. STRAUSS /Director, Research & Development Burgess Battery
Division, Clevite Corp.
After 18 years in battery research and development, the author is considered one of the country's leading authorities on chemical power sources. A graduate of the College of the City of New York, he received his doctorate from Columbia University in 1949. Prior to assuming the position of Director of R & D at Burgess, he was Associate Director for R & D at the Electric Storage Battery Co.
There are almost as many different kinds of batteries as there are applications. Often the engineer needs help in deciding whether mercury, alkaline, or lead-acid cells best meet his needs.
IT wasn't too many years ago that battery manufacturers matte only LeClanche cells ( the type used to power flashlights) and lead-acid batteries for automobiles. There were some other electrochemical systems, such as nickel-iron (Edison) batteries for traction work, copper-oxide (Lalande) batteries for railroad signaling, and air depolarized cells for telephone and telegraph applications, and so on. But, by and large, these units had little commercial importance anti were, even by contemporary standards, of rather simple design.
Burgeoning Business
But since World War II, the demand for new battery systems has skyrocketed and there have been significant engineering advances in some areas of battery manufacturing. But space. military requirements. and the needs of other modern electronic systems, have pushed the development of new batteries until today we are utilizing power devices that are literally out of this world. But the requirements grow still more demanding.

Table 1. Fundamental cell characteristics.
Battery manufacturers have indeed risen to this challenge and. in fact, we now have in commercial production a large variety of battery systems in various sizes and operational and environmental performances. As a matter of fact. one of the big problems now is that there are so many batteries the electronics designer has difficulty in choosing the best unit.
Usually, the problem is compounded because the electronics engineer knows little of electrochemistry and is not in a position to make an independent choice.
Unfortunately, while many manufacturers have gone to the trouble of listing battery potentials. the proper choice of a battery supply often boils down to how the system acts under certain operating conditions. Basically. it's quite easy to calculate how mach energy a battery mast provide. and the power level at which this energy must be supplied. Most battery systems are able to provide almost any energy output at almost any power level since, after all, a suitable combination of cells in series and parallel will deliver the necessary voltage and current for any desired length of time. Determining which battery system to use is. therefore. the result of an intricate study of the second-order type of selection. namely. space and weight, environmental conditions, temperature and pressure, stress relationships, and so on.
In making his selection, the electronics designer should be intimately familiar with the conditions under which his apparatus (in this case, the power supply) is to work.
Usually, however. it is late in the development cycle when the operational parameters are pinpointed. Thus, many electronics designers find themselves boxed in since the space and weight requirements may have been fixed without any consideration being given to the requisites of a particular battery-power source.
Selecting a Battery
It is best that electronics designers consider, right at the outset, the basic power source requirements and make a preliminary selection of a battery system which could be used in the equipment. To help the engineer, Table 1 lists sixteen fundamental cell characteristics which should be considered in the selection of a battery power supply.
Some of these, such as energy per unit weight and energy per unit volume (Items 1 and of the table) are fairly straightforward in that the electronics designer need only match the amount of energy required with space and weight limitations. Of course, the rate at which this energy is to be used is also of paramount importance, but the table does differentiate between sustained and pulsed power levels (Items 3 and 4) . It turns out that batteries can deliver tremendously high power if they are required to provide such power only for extremely short duration.
Thus, power loads must be broken down into sustained power and the power needed in short bursts. Generally speaking, power bursts of short duration may be 100 times that which the battery will sustain over a prolonged period of time. In other words, it's possible for low-power, long life batteries to deliver tremendous power levels for extremely short durations.
Good shelf life or good charge retention (Item 5) is self-explanatory, but good voltage regulation (Item 6) requires some comment. Voltage regulation, as applied to batteries, is the property of delivering energy at constant voltage. Most batteries, in fact all of them with but one significant exception, have open-circuit voltages which are very much higher than their operating voltages. Thus, in electronics circuits which use multi-cell batteries, voltages are much higher at the beginning of the operating cycle than they are during the balance of the operating period. When the circuit is sensitive to the power-source voltage or when high initial power bursts are detrimental, this is a very important design consideration.
The remaining characteristics (Items 7 through 16) are all essentially self-explanatory. However, it should be pointed out that not all of these characteristics are of equal importance in every case. In the development of a flashlight, for example, the availability of a battery like that in Item 16 is of paramount importance. It wouldn't be to anyone's advantage to own a flashlight for which one had to ask a manufacturer to design a special replacement battery.
To help the electronics designer select the most suitable battery system, Table 2 lists the major commercially available battery types and rates them in terms of the sixteen fundamental cell characteristics of Table 1. A rating of "good ". "fair ", or "poor" has been assigned for each of the cell systems. For example, mercury cells are listed as "good" as far as energy per unit weight and volume, shelf life, and voltage regulation are concerned. In addition, mercury cells are quite good in that they require minimal maintenance and are easy to store. They also have high reliability and good mechanical integrity, are pleasing in appearance, and are conveniently available. On the other hand, they are only "fair" in their power output capability and their ability to withstand high temperatures, and are reasonably expensive. Therefore, when we consider the battery's pulse power capability and low temperature characteristics, mercury cells are not too impressive.
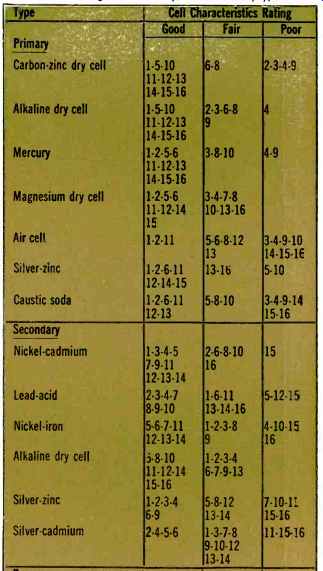
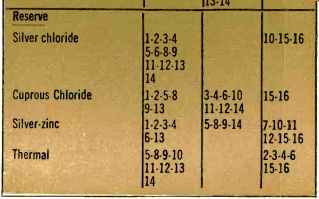
Table 2. Listing of commercially available battery types.
Needs vs Capacity
Naturally, the battery can be designed so that any one or several of these properties can be enhanced, but this is usually at the expense of some of its other capabilities.
What is important is that this tabularized information represents an over-all summary, and to some extent opinion, of battery systems which the designer can utilize.
However, the designer should remember that the tables represent only very general information and that the information can be considered biased under certain conditions. For example, what is high cost to one electronics designer may indeed be insignificant to another.
Current battery technology is receiving a great deal of academic and industrial attention. While battery suppliers have clone a good job in providing designers of electronic (and other types of portable electrical gear) equipment with well-designed power sources, they have presented him with a perplexing array of batteries which require careful study. Considerable attention must be given to achieving a suitable balance between the available cell and their characteristics.