By SIDNEY L. SILVER

Our technology is expanding but the quality of our air continues to deteriorate as more and more pollutants are poured into the atmosphere, Types of pollutants, standards, and electronic methods of monitoring and control are covered.
IN recent years, there has been a rapidly growing international concern about the quality of the human environment. Air pollution, for example, is generally recognized as one of the most significant and challenging problems of modern society. It is a paradox that with an advancing and expanding technology, the quality of the air continues to deteriorate as more and more pollutants are poured into the atmosphere.
The fear exists that the spread of air contamination is so rapid that society may suffer irreversible effects before the destructive capabilities of various pollutants are firmly established. For this reason, the task of tracking down, isolating, and eliminating the important air contaminants calls for an interdisciplinary effort involving the collaboration of many trained persons in various fields of applied science and technology. Among these areas are electrochemistry, optoelectronics, toxicology, meteorology, and the nuclear sciences.
Nature of the Problem
Air pollution is a complex and diverse problem, the nature and seriousness of which can vary from one place to another, from season to season, and even from hour to hour.
It may be broadly defined as the presence in the ambient air of one or more pollutants in such quantity and of such duration as to be injurious to human, animal, and plant life, or to property; or as to interfere unreasonably with the comfortable enjoyment of life and property. These pollutants consist of foreign matter suspended in the atmosphere in the form of smoke, vapor, mist, or dust particles which can adversely affect the environment by producing undesirable changes in the physical, chemical, or biological characteristics of the air.
As a rule, individual contaminants do not exist alone in the air but are intermixed with other pollutants at various concentration levels. These substances sometimes react with each other. e.g., in the presence of sunlight, to produce new and sometimes unknown compounds. There are other meteorological variables, such as wind direction, wind velocity, and air-temperature variations with altitude, which influence the transport and dispersal of air pollutants, resulting in the subsequent dilution of concentration levels. Topography also plays a role in creating a localized atmospheric system, thereby affecting the air quality. In big cities, for example, the many huge structures rising to different heights and arranged in various patterns, provide a large surface for the absorption of more solar energy into the urban atmosphere.
An important factor is the cause-and-effect relationship between air contamination and health, namely, the specific mechanism by which this phenomenon can produce disease. While statistical evidence indicates that adverse health effects are most common in communities having the greatest concentration levels, no correlation can be made to show that any single pollutant is the cause of these symptoms. Moreover, the health effects of less intense pollution exposures over long periods of time are not known. At the present time there is insufficient reliable, quantitative information concerning the "tolerable levels," i.e., the specific measured point at which normal persons exposed to an identifiable pollutant will experience no adverse reactions or physical impairment.
Sources of Air Pollution
The contamination of the atmosphere is caused by various natural phenomena, as well as human activity. Some of the natural forces that create pollution are volcanic eruptions, earthquakes, some forest fires, and natural radioactivity. This article, however, will deal specifically with manmade or artificial air pollution which derives mainly from the everyday activities of the inhabitants in various communities.
In urban and industrial areas, the primary sources of air pollution are the combustion processes involving fossil fuels (mainly coal and oil) for the generation of power, space heating, the processing of materials, as well as the burning of waste products. The principal mobile source of pollution is the gasoline-powered motor vehicle. Although the exhaust emissions of other modes of transportation such as locomotives, airplanes, and ships may be significant locally, they do not add much to the community-wide air pollution.
As a result of these operations, about 142 million tons of pollutants are released into the atmosphere in the U.S. each year; the most common of which are carbon monoxide, the sulfur oxides, hydrocarbons, particulate matter, and the nitrogen oxides. Fig. 1 indicates the average concentration levels annually.
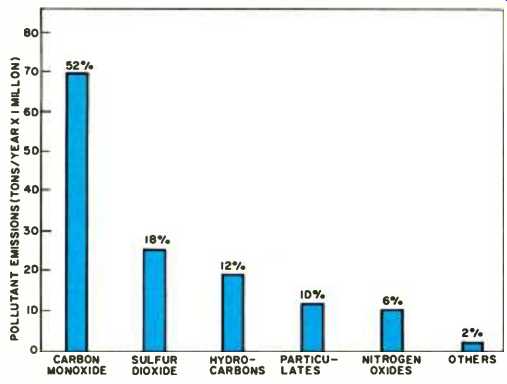
Fig. 1. Pollutants discharged into atmosphere over U.S. yearly.
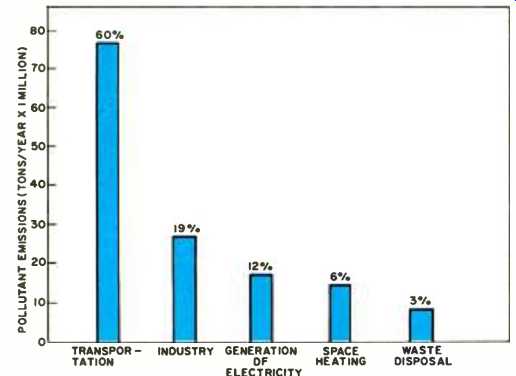
Fig. 2. Sources of pollutants in atmosphere over U.S. annually.
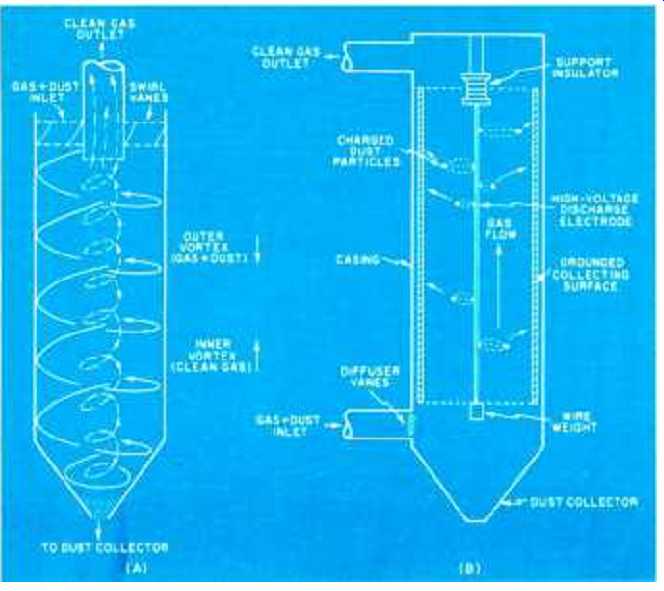
Fig. 3. (A) Cyclone separator uses centrifugal force to collect dust.
(e) Cutaway of tubular electrostatic precipitator.
The contribution of various sources during the same period is shown in Fig. 2. It can be observed that the carbon monoxide (CO) level exceeds that of all other contaminants combined, and thus can be regarded as one of the prime indicators of air pollution. Carbon monoxide is an invisible, toxic gas pollutant which comes mostly from fuel that is not completely oxidized to carbon dioxide and water; motor-vehicle exhausts accounting for over 90% of the total CO emitted into the atmosphere. The hydrocarbons are another class of pollutants which emanate as unburned, or partially burned gaseous compounds formed during the high-temperature combustion process.
Still another product of the fuel-burning process is nitrogen oxide (NO), chiefly contributed by internal combustion engines. NO is formed because of the very high temperature dissociation of molecular nitrogen and oxygen from the intake air used to burn the fuel. After emission into the atmosphere, the NO is converted to nitrogen dioxide (NO2) which, in turn, reacts with other contaminants in the air to produce a variety of toxic substances. For example, the interaction of a dilute mixture of NO2 and hydrocarbons produces complex chemical changes during the sunlight hours, resulting in the formation of photochemical smog.
The second most prevalent gaseous pollutant in the atmosphere is sulfur dioxide (SO2), produced mainly by the combustion of coal and oil which contain appreciably large quantities of sulfur as an impurity. Owing to catalytic action with other materials in the ambient air, the SO2 is more or less oxidized to form sulfur trioxide (SO3) which, in turn, reacts with water vapor to yield a dilute but corrosive mist of sulfuric acid (H2SO4). Besides the pollutant gases dumped into the atmosphere, there are the visible emissions caused by the presence of fine solid and liquid particles, or particulate matter, mainly discharged by industrial smokestacks. These particulates are composed of a wide range of substances, including dust, soot, grease, mineral matter, and microscopic particles of metals and metal oxides. Some of these particles are large enough to settle rapidly toward the earth, but many others are sufficiently small to remain suspended indefinitely in the ambient air until they are removed by wind or precipitation.
Control Techniques
Technology exists today for controlling and reducing most of the pollutants that stationary sources would otherwise release into the atmosphere. The primary function of any pollution-control device is the removal and neutralization of particulate matter and gaseous material. Generally, the particles on which the gas pollutants have been absorbed are separated by particulate collectors, and then the gases are either reduced to harmless substances or recovered by other methods.
In the case of particulate matter, particle size distribution and the amount of dust involved are critical parameters because they determine selection of the proper control device. The common denominator used in referring to particle size distribution is the micron (one-thousandth of a millimeter), with atmospheric dust particles ranging from hundreds of microns in diameter down to almost molecular dimensions. Usually, particles larger than 50 microns do not remain airborne for long periods of time unless there is considerable air turbulence.
The simplest method of reducing stack emissions is the cyclone collector which depends upon the property that particulates have greater inertia than gaseous substances.
In operation (Fig. 3A), the dust-laden gas is forced into a cylindrical tube through swirl vanes which induce a high-velocity spiral action to the gas-dust mass. Owing to centrifugal force, the particles are flung to the walls of the chamber and subsequently carried down by gravity to a dust outlet. At the bottom of the chamber (usually terminated in a cone), the clean gas stream reverses its vortex and flows upward through a center exit port. Devices of this type achieve a high collection efficiency with particles in the 10 to 200 micron range. Sometimes wet inertial devices, or "scrubbers," are employed in which high-pressure jets of liquid are able to extract gaseous pollutants from particulate matter.
Another way to remove dust from process gases is by filtration, where particulates are trapped by a large number of fabric filters, while the dust-free gas passes through the filter to an outlet. For large particles, the inertial impaction of the dust on the filter fibers is the predominant collector mechanism. However, as the dust accumulates, the submicron particles are actually sieved from the gas by molecular diffusion. The resistance of the gas flow to the collected material eventually increases so that the filter must be cleaned periodically by subjecting the system to mechanical vibration.
At the present state of the art, the most widely used high-efficiency collector of particulate matter is the electrostatic precipitator. As shown in Fig. 3B, the basic device consists of a tubular collecting surface placed at ground potential, with a discharge electrode centered along the longitudinal axis. The center electrode is energized with a high negative potential (on the order of 100 kV peak) so that a corona discharge is established around the electrode. As the gas-dust mass passes through the corona the gas is ionized, and these ions migrate toward the collecting surface where they collide with the suspended dust particles. During the ion bombardment, the dust particles take on a negative charge and drift in the direction of the grounded collector, where they adhere until removed by electromagnetic vibration. These devices have a high efficiency in the collection of small particles in the 0.01- to 1-micron range.
The removal of gaseous pollutants may sometimes be accomplished by oxidation, whereby combustible process gases are recycled into the burning chamber. Gases which are more or less soluble in water may be removed by absorption. Here very small droplets of water (containing certain chemicals to enhance solubility) present a high surface area to the gas stream so that the gas dissolves or reacts across the gas-liquid interface. In the adsorption method (Fig. 4), the pollutant gas is forced to adhere to the surface of activated carbon material where the molecules adsorbed from the gaseous state are collected into a condensed layer. The carbon is then regenerated with low-pressure steam, and the resultant steam-vapor mixture is cooled in a condenser unit, then fed to a separator where the gas is recovered.
For some pollutants, such as the sulfur oxides, no completely satisfactory method of removal has yet been fully developed. Obviously, the removal of sulfur from coal prior to burning would be the ideal solution, but this is not feasible at present because most of the sulfur is organically bound to the coal and can only be released by combustion. One process which has been developed involves the injection of powdered limestone into the combustion chamber, thereby producing reactive compounds which combine with and eliminate some of the SO2. Alternate means of control include the use of low-sulfur coal, de-sulfurized oil, and natural gas.
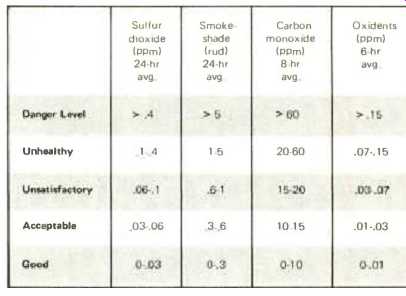
Table 1. Criteria of dally air-pollution index for N.Y. City.
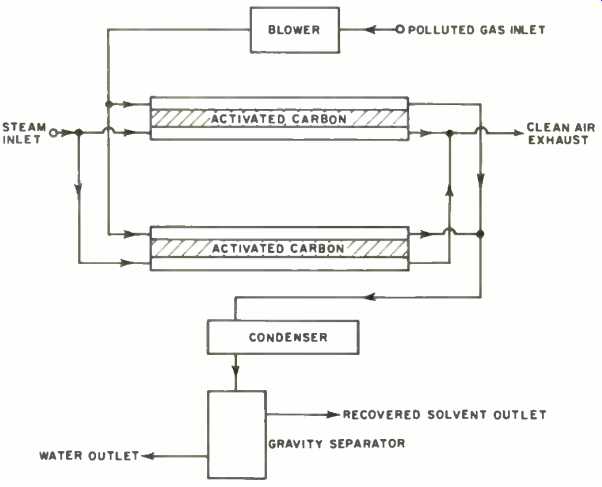
Fig. 4. A flow diagram of a typical adsorption system.
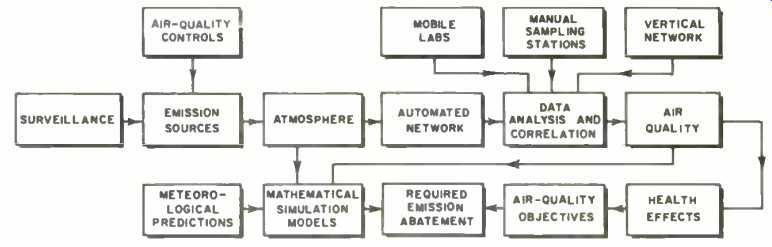
Fig. 5. The operation of an air-quality monitoring and control system.

Fig. 6. Technician checks the readings for smoke-shade at one of New
York City's ten automated telemetry stations.
---------
Table 2. Control limits for various air-pollution conditions.
1. Forecast Stage: Meteorological conditions indicate that a high pollution potential will exist for the next 36 hours.
2. Alert Stage: Stage is reached if for any consecutive 6 of the previous 12 hours:
1. Forecast predicts that adverse weather conditions (stagnation) will continue for 12 more hours.
2. Sulfur-dioxide exposure = 2 ppm-hrs
3. Soiling-index exposure = 2 rud-hrs
1. Carbon-monoxide exposure = 180 ppm-hrs
3. Warning Stage--Stage is reached if for any consecutive 6 of the previous 12 hours:
1. Forecast predicts that stagnation will continue for 12 more hours
2. Sulfur dioxide exposure = 3 ppm-hrs
3. Soiling-index exposure = 25 rud-hrs
4. Carbon-monoxide exposure = 300 ppm-hrs
4. Emergency Stage: Stage is reached if in a 24-hour period:
1. Forecast predicts that stagnation will continue for 12 more hours
2. Sulfur-dioxide exposure = 15 ppm-hrs and is rising
3. Soiling-index exposure = 200 rud-hrs 44
-----------
The reduction of air pollution produced by motor vehicles requires improved systems for controlling the emissions of hydrocarbons, carbon monoxide, and the oxides of nitrogen. Hydrocarbons and CO emissions may be reduced by the injection of air into the exhaust manifold at the point of hottest gas temperature, in order to continue the combustion of these pollutants in the exhaust system. Since gasoline is highly volatile, the gas tank and carburetor lose hydrocarbons through evaporation, these vapors also contributing to air pollution. To offset this problem, the system may incorporate a liquid-vapor separator in the fuel system, with a line returned to the carburetor to permit the purging and burning of stored vapors. The control of NO emission may be achieved by automatically regulating the spark advance and controlling fuel combustion so that peak burning temperatures are reached at lower levels.
Although a sharp reduction in pollutant emissions will probably be attained during the next decade, attention must be given to new approaches involving propulsion systems with inherently low emission characteristics. These include such power systems as turbine and steam engines where the levels of hydrocarbons and CO emissions are considerably lower. Electrically propelled vehicles appear to offer some potential as pollution-free devices, but further advancement in battery or fuel-cell technology will be required before acceptable performance is achieved.
Air-Quality Standards
In setting standards, the quantitative relationship between the contamination of the air and the sources of emission is difficult to determine, since there are usually many sources which emit similar or identical pollutants. Ambient air standards are concerned with the concentration of pollutants present in the atmosphere, while emission standards apply to the quantities of pollutants discharged from specific sources.
In order to deal systematically with these problems in the U.S., the Air Quality Act of 1967 was developed and passed to strengthen the control effort. This law builds on earlier legislation going back to 1955, and is in the form of an amendment to the Clean Air Act of 1963. Accordingly, the Department of Health, Education, and Welfare (HEW) has designated specific air-quality control regions that cover various communities sharing a common air-pollution problem.
HEW has also developed and published a number of air-pollution criteria indicating the extent to which a specific pollutant, or a combination of pollutants, is harmful to health or damaging to property. These criteria reflect the best available scientific data on the effectiveness of existing technology for the prevention and control of air contamination. As soon as the control techniques for a particular pollutant are issued, each state involved in the region is given the responsibility for developing air-quality standards and plans for implementing them.
An important section of the 1967 law provides for a comprehensive study of the need for, and effect of, national emission standards for stationary sources of air pollution. As a consequence, to broaden the scope of the law, the House and Senate have recently passed separate bills to establish national air-quality standards. In the Senate version, the national standards would cover 10 major contaminants, and would prohibit all emissions of a hazardous nature not covered by the standards. The bill would further require that the 1975-model automobiles achieve a 90% reduction of present-day standards for emissions of carbon monoxide, hydrocarbons, and the nitrogen oxides. As of this writing, both bills are before a Senate-House conference committee to resolve the differences between the two measures.
As an example of how the Federal criteria are applied, Table 1 shows the daily air-pollution index for the New York City area. Here the numerical values and time spans are given for four of the most common pollutants. These designations, covering various concentration levels, relate to the New York State standards which, in turn, are based on the health-effects criteria developed by HEW. In the case of sulfur dioxide, for example, the designation "good" (corresponding to the range from 0 to 0.03 parts per million) has been set as the annual average goal for that area because there is no scientific evidence of any adverse health effects within this range. The criteria further state that the 24-hour average concentration of sulfur dioxide should not rise above 0.1 ppm (to the "unhealthy" range) more than about three times a year. It should be pointed out that the objectives set forth in these criteria will be immediately subject to revision if further scientific investigation reveals any new health hazards associated with air-pollution levels.
The standards applied to carbon monoxide suggest negligible health effects below 10 ppm when levels are averaged over an 8-hour period. Since concentrations rarely reach this magnitude on a city-wide basis, the measurement is regularly taken in high-traffic areas where the levels are high at breathing level.
Smoke-shade, sometimes referred to as the soiling index, is a measure of the dust, grime, and particulate matter in the ambient air, and is expressed in terms of the reflectance units of dirt-shade (rud). Here the range of levels covers the average city-wide concentrations over a 24-hour period.
The oxidants refer to a group of toxic photochemical compounds, e.g., ozone, formed by the action of sunlight on the oxides of nitrogen and hydrocarbons. These levels are averaged over a 6-hour period during the time of strongest sunlight.
In order to minimize the impact of air-pollution episodes, such as may occur during periods of stagnant weather, air-quality control limits must be established, These limits are usually stated as a concentration level that should not be exceeded when either averaged or integrated over a predetermined time interval. These serve as a reference value for triggering control action when the measured air quality has deteriorated to a certain point. In Table 2, a representative set of control limits is given for New York City, indicating the essential steps for implementing an alert-warning system.
Air-Quality Monitoring Systems
In order to evaluate the effectiveness of a pollution-control program and determine what corrective actions are required, sophisticated monitoring systems are employed in a number of metropolitan regions to provide a detailed profile of air pollutants.
Fig. 5 shows a block diagram of an advanced air-quality monitoring system, or aerometric network, which enables a computer-controlled central processing station to determine whether specified pollution levels have been reached. In this network, major traffic areas and smoking chimneys are constantly under surveillance by mobile vehicles capable of communicating information to the central station. To provide a vertical profile of concentration levels, the system may include detection equipment mounted in towers and tall buildings, as well as an instrumented helicopter for monitoring the common pollutants and weather patterns in the upper atmosphere. If the helicopter is equipped with TV facilities, the operator at the control panel continuously scans the skyline for visible signs of smoke emissions. If he spots a potential pollutant emitter, he can activate a zoom lens on the camera to confirm his initial observation, thus identifying the offending building.
To minimize "smoke-chasing" by field personnel and increase the effective control over emission sources, on-site air-sampling devices may be installed on the smokestacks of major pollutant producers to measure the rate of flow of pollutant stack gases and to extract a sample of the gas. The telemetered information gathered from these air samplers is then fed instantaneously over telephone lines to the central control station for recording and analysis. Stack-monitoring systems, however, are generally exposed to very high temperatures within the stack (on the order of several hundred degrees F), so that these analytical instruments must be designed to withstand the severe environment. In more advanced systems, the use of electro-optical devices permits remote analysis of gaseous pollutants from an emitting source.
Here a telescope is focused on the smoke plume in question and the hot pollutant gases are identified and measured using infrared analyzers.
In addition to the measurement of pollutant concentrations an essential input to any air-quality control system is a meteorological program to accurately profile the particular pollution problem. For this purpose, a number of automated telemetry stations are used at strategic locations to measure wind velocity, wind direction, and air temperature; as well as concentration levels of carbon monoxide, sulfur dioxide, suspended particulate matter, and dust fall.
Fig. 6 shows a typical automatic station forming part of New York City's aerometric network. Supplementing these operations are a number of manual stations equipped with probes to collect samples of the common pollutants, these samples being returned to the laboratories for subsequent analysis. The aerometric network thus delivers a large quantity of raw data to the central station, where a digital computer provides a rapid assessment of the prevailing air quality throughout the monitored areas. Periodically, the computer automatically interrogates each remote station and records the incoming pollutant information, the averaged data being formulated for paper punch, teleprinter, or computer entry.
The final stage of the control system includes the introduction of prediction methods involving both the expected pollution emissions and weather forecasting, so as to anticipate the concentration levels in different areas for a specified period, say 24 or 48 hours.
Here the total pollution information and meteorological data are combined into a mathematical simulation model designed to interpret the over-all air quality. Given sufficient data, the control agency can then decide whether or not to initiate emergency control measures.
Ideally, air-quality control limits should also be based on an analysis of the medical effects of air pollution, including a calculation of the minimum time of exposure to various pollutants that are likely to cause certain symptoms. If the situation is considered to be hazardous to health, for example, then it is important to predict the short-term rise in pollutant concentrations, clearly a more difficult problem than predicting the extended levels.
On the international level, the United Nations Conference on the Human Environment will be convened in 1972 to consider the global effects of all types of pollution. Among the objectives will be an attempt to organize the pollution-control effort through multi-lateral action by many nations. This should lead to the establishment of a global network for monitoring pollutants, and provide a basis for the subsequent formulation of world-wide air-quality standards.
======
Which Computer--The Programmable Calculator?
======
(adapted from: Electronics World magazine; Jul. 1975)
================